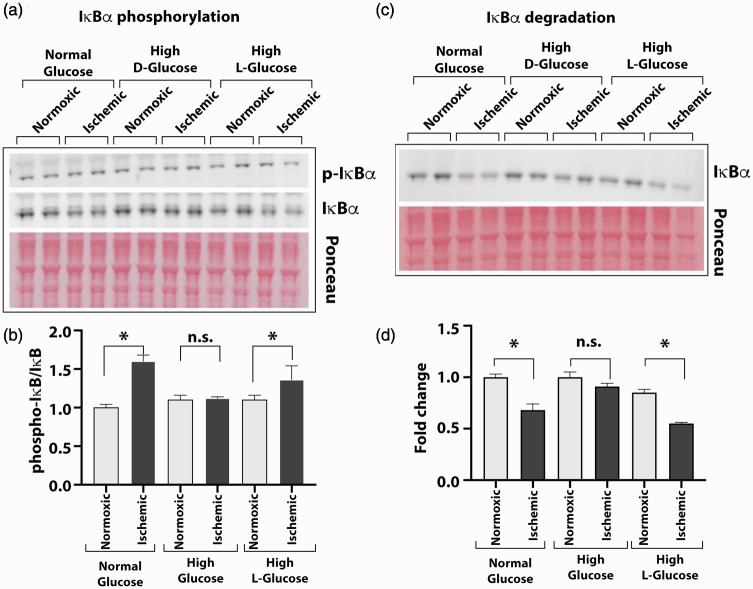
Figure 3.
High glucose exposure attenuates the ischemia-induced NF-κB activation. HUVEC were grown in normal (5 mM) or high D-glucose (25 mM) containing culture medium before exposing them to ischemia and analyzing cell lysates for IκBα phosphorylation and degradation to measure the activation of NF-κB. Control cells were also grown in high concentration of metabolically inert L-glucose (25 mM) in these experiments. (a) Western blots of phosphorylated (p-IκBα) and unphosphorylated (IκBα) forms of IĸBα protein from cells grown in normal, high D-glucose, or high L-glucose with normal (normoxic) or ischemic treatments. (b) Intensity of protein bands on Ponceau S-stained blots was used for loading control. Protein quantification of IĸBα was done by densitometry, and results are presented in the bar graph as the ratio of p-IκBα to unphosphorylated form. (c) Degradation of IκBα was tested by Western blot of the HUVEC cultures in normal or high glucose conditions using specific antibody. Signals from Ponceau S-stained the blot were used for loading controls. (d) The ratio of IκBα to total protein is presented in the bar graph. Pair-wise comparisons were done between normoxic and ischemic samples in each group for statistical analyses. Asterisks denote P < 0.05, and n.s. denotes not significant; n = 5 to 14 in each group. (A color version of this figure is available in the online journal.)