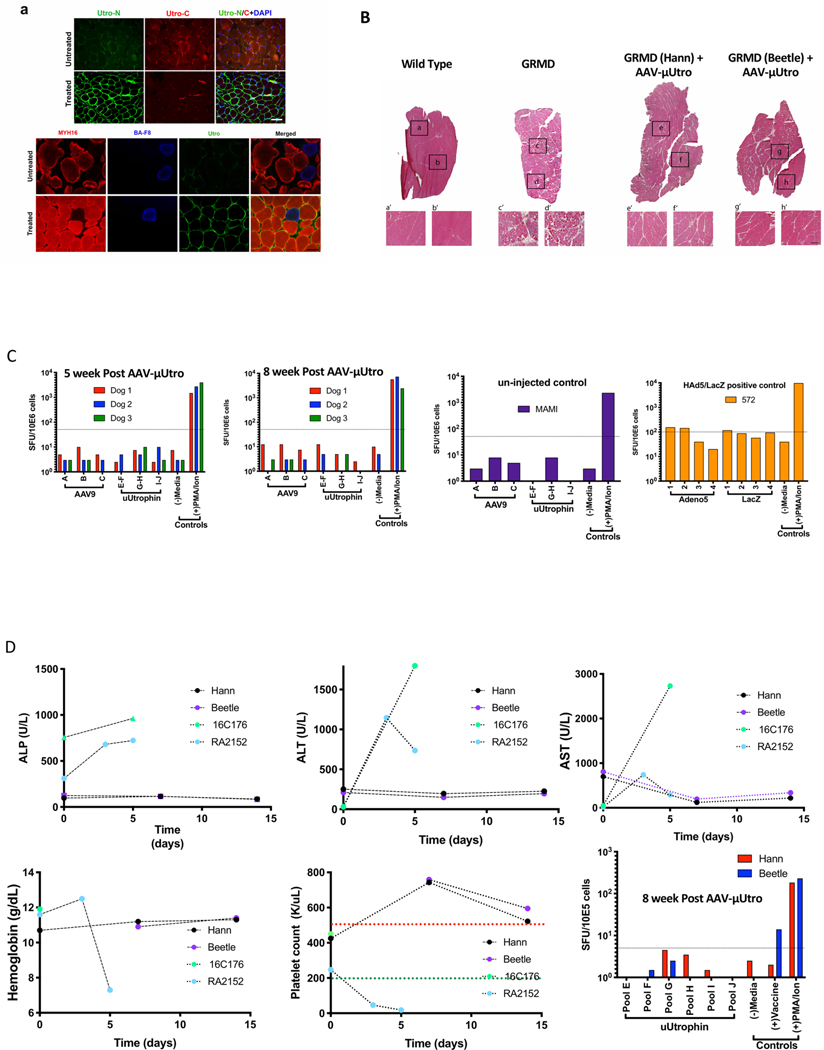
Extended Data Fig. 8 |. μutro expression provides histological improvement without any signs of toxicity in injected GRMD dogs.
a, Representative images of temporalis muscle from age-matched AVV-μUtro (treated) and PBS (untreated) GRMD dogs stained against an N-terminal epitope shared by native and recombinant utrophin (Utro N) and a C-terminal epitope unique to native utrophin (Utro C) (top) and MYH16, BA-F8 (slow twitch myofiber) and Utro N (bottom). Scale bar, 20 μm. Experiment was repeated independently at least two or more times with similar results. b, H&E stain. Top: A whole view of vastus lateralis muscle obtained from a muscle biopsy four weeks post-injection. Botom: A higher magnification of the corresponding boxed region. The experiment was repeated independently at least two times with similar results. c, γ-Interferon production was quantified by counting the spot forming units (SFUs) per million peripheral blood mononuclear cells (PBMCs). A response above the dotted line is considered positive and above the background signal. Peptide libraries used represent the full sequence of AAV9 capsid (A–C), μUtro (E–J), Adenovirus5 (1–4) and LacZ (1–4). d, Measurement of alkaline phosphatase (ALP), alanine aminotransferase (ALT), aspartate aminotransferase (AST), hemoglobin, platelets and γ-interferon before and after AAV-μUtro injection in two juvenile GRMD dogs (Hann/Beetle). Non-human primate (16C176 and RA2152) data are incorporated to illustrate the absence of disseminated intravascular coagulation (DIC)33,34. (See Source Data Extended Data Fig. 7).