Fig. 2 |. Formulation with CB[7]-PEG stabilizes a co-formulation of Novolog or Humalog and pramlintide at physiological pH.
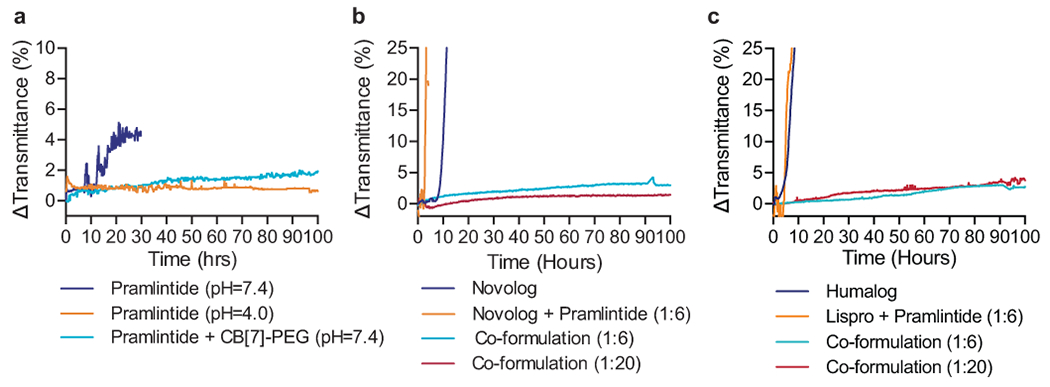
a, In vitro stability of pramlintide formulations at various pH values with and without CB[7]-PEG. b, In vitro stability of pramlintide-aspart (1:6 and 1:20 molar ratio) co-formulations with CB[7]-PEG at physiological pH. c, In vitro stability of pramlintide-lispro (1:6 and 1:20 molar ratio) co-formulations with CB[7]-PEG at physiological pH. Co-formulations were compared to controls of commercial Novolog or Humalog, and mixtures of the incompatible aspart+pramlintide or lispro+pramlintide in the absence of CB[7]-PEG. These assays assess the aggregation of proteins in formulation over time during stressed aging (i.e., continuous agitation at 37ºC) by monitoring changes in transmittance at 540nm. These experiments demonstrate that formulation with CB[7]-PEG prevents protein aggregation over the 100h period assayed, even when commercial formulations aggregate within 10h. Data shown are average transmittance traces for n = 3 samples per group.
