SYNOPSIS:
It is generally accepted that up to 50% of those with a whiplash injury following a motor vehicle collision will fail to fully recover. Twenty-five percent of these patients will demonstrate a markedly complex clinical picture that includes severe pain-related disability, sensory and motor disturbances, and psychological distress. A number of psychosocial factors have shown prognostic value for recovery following whiplash from a motor vehicle collision. To date, no management approach (eg, physical therapies, education, psychological interventions, or interdisciplinary strategies) for acute whiplash has positively influenced recovery rates. For many of the probable pathoanatomical lesions (eg, fracture, ligamentous rupture, disc injury), there remains a lack of available clinical tests for identifying their presence. Fractures, particularly at the craniovertebral and cervicothoracic junctions, may be radiographically occult. While high-resolution computed tomography scans can detect fractures, there remains a lack of prevalence data for fractures in this population. Conventional magnetic resonance imaging has not consistently revealed lesions in patients with acute or chronic whiplash, a “failure” that may be due to limitations in the resolution of available devices and the use of standard sequences. The technological evolution of imaging techniques and sequences eventually might provide greater resolution to reveal currently elusive anatomical lesions (or, perhaps more importantly, temporal changes in physiological responses to assumed lesions) in those patients at risk of poor recovery. Preliminary findings from 2 prospective cohort studies in 2 different countries suggest that this is so, as evidenced by changes to the structure of skeletal muscles in those who do not fully recover. In this clinical commentary, we will briefly introduce the available imaging decision rules and the current knowledge underlying the pathomechanics and pathophysiology of whiplash. We will then acknowledge known prognostic factors underlying functional recovery. Last, we will highlight emerging evidence regarding the pathobiology of muscle degeneration/regeneration, as well as advancements in neuroimaging and musculoskeletal imaging techniques (eg, functional magnetic resonance imaging, magnetization transfer imaging, spectroscopy, diffusion-weighted imaging) that may be used as noninvasive and objective complements to known prognostic factors associated with whiplash recovery, in particular, poor functional recovery.
Keywords: cervical spine, functional magnetic resonance imaging (fMRI), magnetic resonance imaging (MRI) research, radiology/medical imaging, spinal pain
It is generally accepted that up to 50% of those with a whiplash injury should expect to recover within the first 2 to 3 months following a motor vehicle collision (MVC). Accordingly, approximately 50% will not fully recover,15 of whom approximately 25% will demonstrate a markedly complex set of signs/symptoms that include severe pain-related disability,113 changes in the structure of neck muscle,29,32,34,39 sensory and motor disturbances,114 muscle weakness,89,108,115 and psychological distress.114,116 Though there is evidence to suggest damage to a number of tissues (facet joints and capsules, the intervertebral disc, ligaments, vascular tissues, osseous structures),21 no definitive structural cause of the wide and varied symptomatology has been realized with available imaging applications.38,83–85,94,95,105 Recent prospective studies from 2 different countries have, however, identified changes in the neck muscle structure (eg, muscle fatty infiltrates [MFIs]) of those with poor functional recovery,32,25 suggesting a core biological contribution to outcomes. While interesting, the precise mechanisms underlying these muscular changes and their defined influence on recovery remain largely unknown.
A number of psychosocial factors (eg, coping, expectations, anxiety, and depression) have demonstrated prognostic value in whiplash recovery.15 However, despite the presence and recognition of these factors, there remains a paucity of best-evidence treatment options to substantially influence the rate of functional recovery.61,67,79
It is important to be aware of a number of emerging mechanistic models for whiplash recovery. These include, but are not limited to, (1) maladaptive beliefs and cognition,120 (2) stress system dysregulation,74,75,123 (3) genetic vulnerability,12,13 and (4) mild injury involving the peripheral and central nervous systems.32,34–36,40,42,128–130
This clinical commentary will not refute the multifactorial nature of whiplash-associated disorders (WADs)26 or the large prevalence and distribution of abnormal findings on cervical spine magnetic resonance imaging (MRI) in asymptomatic populations.86 Nor will it attempt to sway the reader into believing that early and serial imaging should become a part of standard of care for all patients exposed to, and presumably injured in, a whiplash event. Rather, the present commentary will highlight previous and preliminary findings to support the use of neuromasculoskeletal imaging applications (eg, fat-water MRI, diffusion-weighted MRI [DW-MRI], magnetization transfer imaging, and spectroscopy) as noninvasive and objective complements to other prognostic measures. We will review the important information related to imaging guidelines, as well as introduce technological advances in our understanding of the underlying mechanisms of muscle degeneration/regeneration. We will also introduce and propose the use of advanced, but available, functional magnetic resonance imaging (fMRI) sequences of the brain and spinal cord as having potential scope in measuring the pain experience for patients with whiplash injury. The intention is to facilitate development of productive interdisciplinary collaborations that will propel research in the field of whiplash and WAD into a new era of understanding—and legitimacy on a patient-by-patient basis. Such efforts have potential to generate more efficient management strategies for patients, perhaps interrupting the progression and associated sequelae of chronic pain-related states.
IMAGING CLINICAL DECISION RULES
Clinicians, including physical therapists, are required in the course of routine care to make individual judgments for initial and further diagnostic tests (possibly including imaging) for the patients they manage. This includes not only acutely injured patients presenting to the emergency medicine department with concerns of significant pathology, but also those patients in whom the trajectory of recovery, based on known prognostic factors, is not consistent with a satisfactory outcome.101
The primary guideline in the United States for diagnostic imaging is the American College of Radiology Appropriateness Criteria (ACR-AC).2 The ACR-AC are a set of consensus-developed evidence-derived guidelines for health care providers to assist in decision making for imaging based on apparent health condition or potential condition requiring investigation. The stated goals of the ACR-AC are to enhance the quality of patient care and to contribute to the most efficacious use of radiology. Within the ACR-AC structure, patient presentations are categorized dependent on apparent prior patient history, probable etiology, signs and symptoms, and results of prior imaging. These categories are referred to as “clinical conditions,” with subcategories described as “variants.” Most applicable within the ACR-AC for patients with apparent acute WAD is the clinical condition category of “suspected spine trauma.”22 The criteria for determination of whether imaging is indicated and the recommended modality are based upon the Canadian cervical spine rule (CCSR) and the National Emergency X-Radiography Utilization Study low-risk rule (NEXUS-LRR) (APPENDIX), along with suggestions of neurological or cervical vascular injury.
The CCSR and NEXUS-LRR were developed for the purposes of identifying which patients needed imaging in the emergency department for immediate decision making and those for whom diagnostic procedures were less warranted. The investigations giving rise to these criteria were undertaken because of acknowledgment of negative findings in an overwhelming majority of imaging studies in acute cervical trauma and of the accompanying unwarranted expenses and use of emergency department time and resources.8,44,45,99,104
In the presence of positive clinical assessment findings derived from the CCSR18,117,118 or the NEXUS-LRR,55,91 computed tomography (CT) is the initial imaging modality determined to be “usually appropriate” because of the primary concern for fracture or other destabilizing injury (eg, zygapophyseal joint dislocation) in adults.22
Magnetic resonance imaging is recommended as “usually appropriate” in the ACR-AC suspected spine trauma variants in which neurological involvement or overt ligamentous injury are suspected based on clinical examination, emergent CT results, or if the patient is unevaluable for an extended period (eg, unconscious or obtunded). Clinical suggestions of cervical vascular injury (carotid or vertebral arteries) can include nonspecific symptoms such as neck, occipital, or suboccipital pain or more overt indications of neurological involvement such as vertigo, ataxia, dysarthria, visual field deficit, diplopia, altered cognitive status, and Horner’s syndrome.11,24,25 In cases of suspected cervical vascular injury, either CT or magnetic resonance angiography may be used to evaluate the extent of vessel injury and perfusion by contrast distribution.50,64
It is noteworthy that overt structural or hard neurological clinical findings are rare in the vast majority of patients in the emergent care setting,80,100 which seems to fit with the accepted position that most recovery should occur within the first 3 months following an MVC.15 However, for some patients, recovery is not spontaneous or unremarkable. Whether this discrepancy in recovery rates relates to injury severity or the occurrence of lesions is unknown. What is known is that there is no variant within the ACR-AC guidelines to specifically address the clinical heterogeneity of WAD.1 Accordingly, we contend that future updates to the ACR-AC should consider known risk factors for the heterogeneous WAD condition. In purest terms, most individuals with WAD do not require imaging after receiving a clear medical screen, but some may. This clinical commentary will provide empirical and anecdotal evidence to suggest this is the case and what types of imaging may be most applicable in the acute and chronic stages on a patient-by-patient basis.
Key Points
Published imaging decision guidelines, primarily the ACR-AC, are valuable tools for clinicians assessing patients having experienced acute cervical trauma.
For patients warranting imaging after cervical trauma, CT is the preferred initial modality.
Following cervical trauma, MRI may be warranted acutely with suggestions of neurological involvement or based on the presence of known prognostic factors for poor recovery and for individual patient circumstances in which the soft tissues require detailed assessment.
To date, WAD is not recognized as a specific patient presentation in the ACR-AC; thus, no specific guidelines are available for clinicians evaluating patients having experienced such cervical spine trauma aside from emergent concerns for fracture or instability.
PATHOMECHANICS OF WHIPLASH
Here we provide a brief background regarding the current understanding of the pathomechanics of a typical whiplash injury from an MVC. Engineering-based investigations of MVCs have focused on preventing or reducing the incidence of injury through the modification of the vehicle, including, but not limited to, energy-absorbing seat designs and head-restraint geometry.59,60 Collectively, these studies involving biofidelic dummies, human cadavers, and human volunteers have shown that the total movement of the head and neck, spanning 200 milliseconds during a typical rear-end vector impact, largely remains within physiologic limits,96 but abnormal movements occur within individual segments at upper and lower cervical levels.49 This abnormal movement is referred to as the “S-shape” phase of neck motion (FIGURE 1), which consists of flexion at the upper spinal levels and extension at the lower levels, and can exceed physiologic limits and potentially induce subfailure injuries to a number of tissues (facet joints and capsules, the intervertebral disc, ligaments, vascular tissues, osseous structures).49,62,92,93 There remains equivocal evidence that neck postures or axial rotation at the time of the collision can increase the risk of structural injury,58,112,124,129 but a definitive conclusion likely requires more quantitative “real-world” metrics that currently do not, to our knowledge, exist.
FIGURE 1.
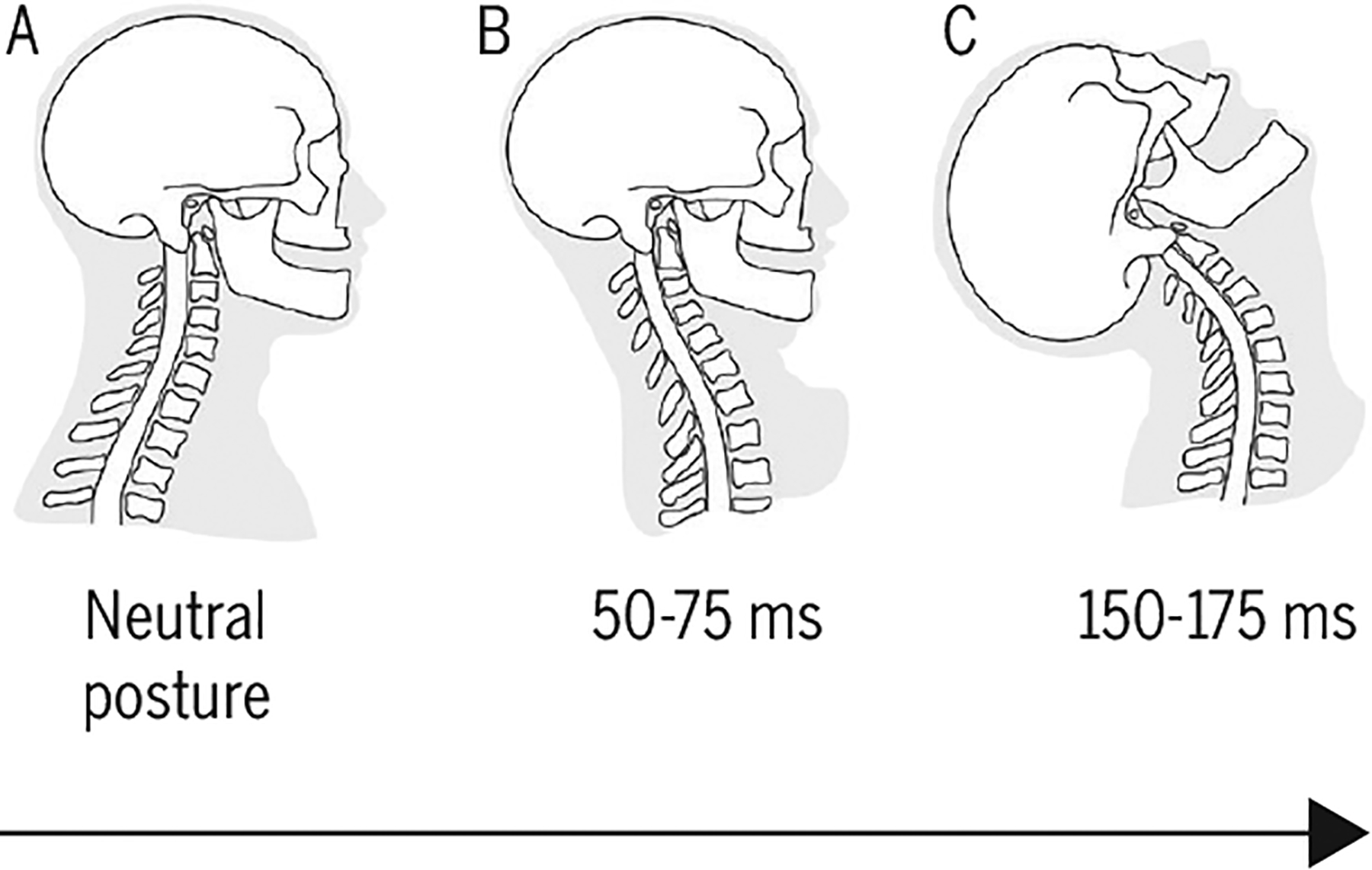
Schematic of the head-neck demonstrating the period during which the cervical spine goes from (A) the neutral posture prior to a rear-end impact to the (B) “S-shaped” curve (50–75 milliseconds). During this time, the upper spinal levels flex and the lower levels extend, exceeding physiologic limits and potentially inducing subfailure injuries to a number of vulnerable tissues. (C) Finally, all of the cervical levels undergo extension, forming a “C-shaped” curve (150–175 milliseconds). Adapted with permission from Grauer et al.49 Copyright ©1997 Wolters Kluwer Health, Inc.
The Elusive Whiplash Lesion and Clinical Practice
Tissue damage following a whiplash injury is evident from (1) laboratory-based applications (simulated crash tests), (2) experimental animal models, and (3) postmortem studies. However, whether or not tissue damage is present in patients with WAD is a clinical, not a laboratory, issue. In purest terms, clinicians evaluate patients to develop an informed and effective treatment plan that addresses potential tissue injuries and identified impairments and dysfunctions on a case-by-case basis.
Unfortunately, to date, there are no radiological means for clinicians to accurately and consistently identify and diagnose tissue injury in the vast majority of patients with WAD. Plain radiography cannot detect soft tissue (eg, facet capsules and discs) damage/injury and even some fractures, particularly at the craniovertebral and cervicothoracic junctions, which may be radiographically occult.70,97 As stated, CT scanning best detects fractures, but to our knowledge, there have been no studies using high-resolution CT to determine the prevalence of fractures following whiplash. Conventional MRI has not revealed structural lesions related to symptoms in patients with WAD, but this may be due to limitations of the image resolution of conventional devices and commonly used imaging sequences. Accordingly, the constant evolution of MRI magnets with improved signal-to-noise and contrast-to-noise ratios might provide greater image resolution, which in the future may reveal currently elusive lesions or physiological responses to damaged tissues.28,29,31–35,39–42
MRI APPLICATIONS
The expression of MFI in the cervical spine muscles in acute and persistent whiplash has been identified in cross-sectional and longitudinal designs across 3 different cultures.29,30,32,35,39,41,63 Such findings were not present to the same magnitude in individuals with persistent idiopathic neck pain,33 or those reporting milder symptoms from WAD.35 Of note, the early and large expression of MFI has recently been shown to occur in those patients35 at risk of poor recovery (higher pain-related disability, older age, and signs of hyperarousal).101 While interesting, the precise mechanisms underlying muscular degeneration (leading to MFI), and their influence on recovery, remain largely unknown. There also remains equivocal evidence to suggest that changes in muscle cross-sectional area are not related to symptoms in the long term.3,71,72,122 This may have more to do with a wide variety of measurement methods, requiring a more concerted global effort to establish a preferred suite of imaging sequences37 whereby pooled analyses can be realized and differences reconciled.
Key Points
To date, no structural cause of WAD has been found with available imaging technology, supporting the position that the clinical course is driven by both medical and noninjury-related factors.
Increased levels of neck MFI on MRI occur in tandem with known predictive risk factors of poor recovery (higher pain-related disability, older age, and signs of hyperarousal).
Routine imaging protocols (CT, radiography, MRI) may need to be reconsidered in the vast majority of patients following whiplash injury.
Etiology of MFI and Cellular Dynamics
It is plausible that functional outcomes in whiplash are, at least somewhat, linked to healthy/unhealthy muscle architecture. Our ability to effectively treat degenerated muscles relies, in part, on an improved understanding of the distribution and etiology of fat deposition across the age spectrum. A major obstacle in the study of MFI is, however, our collective lack of understanding on how, why, and when it occurs, particularly after an injury, making informed disease characterization, longitudinal evaluation, and therapeutic modulation difficult.
What is known? Skeletal muscles have an incredible capacity for repair and regeneration following injury.73 Here, we provide only a general background into repair, as the processes governing this process are complex and beyond the scope of this clinical commentary. For a more detailed description, the reader is encouraged to read the work of either Bentzinger et al9 or Dayanidhi and Lieber.23
The primary myogenic stem cells indispensable for repair and regeneration68 are the satellite cells (SCs).23,125,136 Satellite cells are located in all muscles in their niche, that is, between the sarcolemma of their associated myofiber and the surrounding basal lamina of the extracellular matrix (ECM) (FIGURE 2A). They are in a quiescent (latent) state until activated by any number of stimuli, such as, but not limited to, stretch121 (during growth), the presence of injury and inflammatory factors,136 or denervation27 (after experimental injury). Satellite cells are considered bona fide adult stem cells in that they self-renew106 to maintain the SC pool and are able to create myogenic tissue, that is, muscle progenitors,20 throughout the lifespan. Once activated, the SC proliferates (eg, increase in number) and differentiates (combine with each other) to create nascent myotubes that fuse with and replace the damaged myofibers (FIGURE 2B).
FIGURE 2.
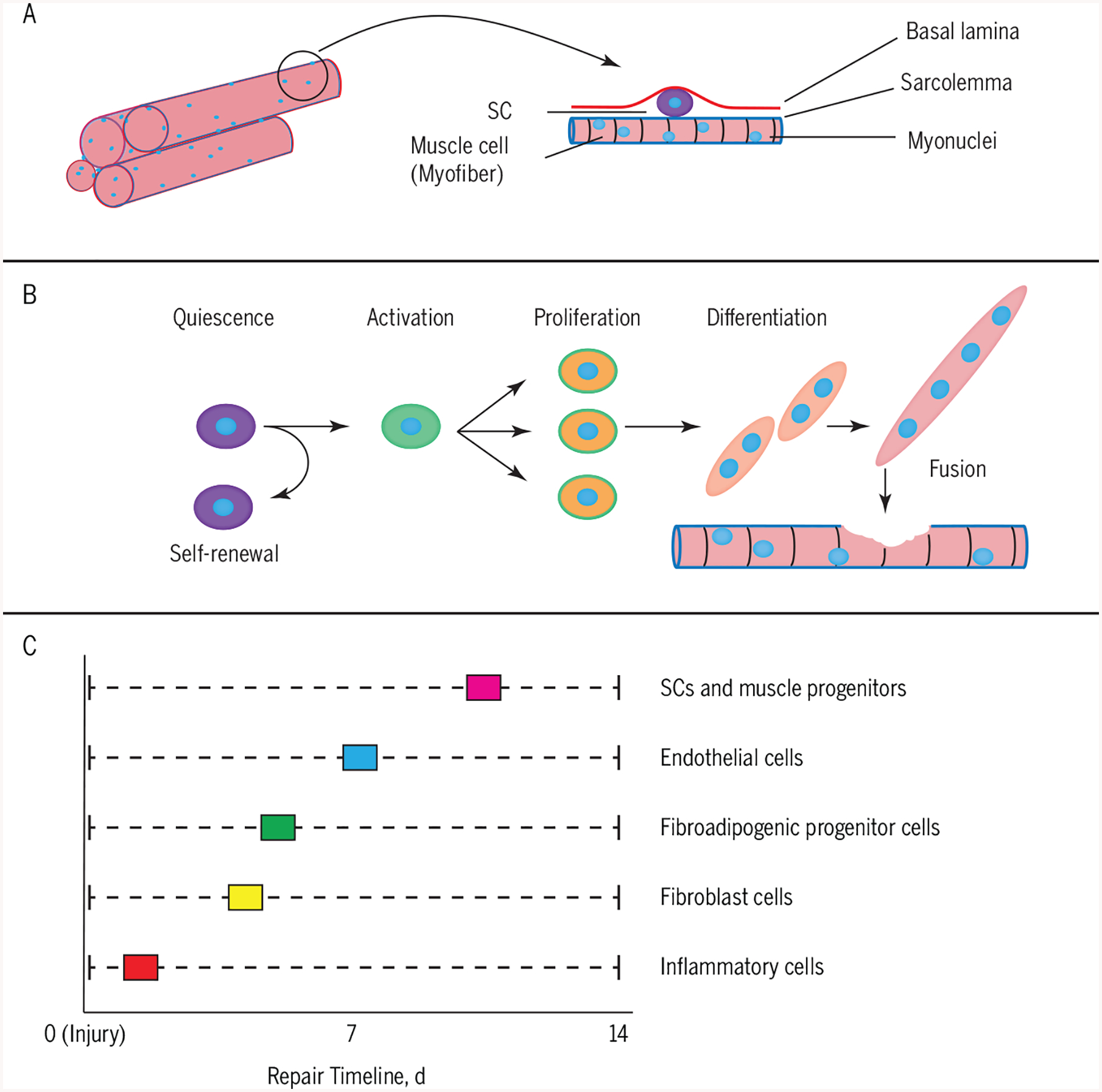
(A) SC niche: SCs lie between the sarcolemma of their associated myofiber and the surrounding basal lamina of the extracellular matrix. (B) SC activation: SCs remain in a quiescent state until activated by a number of stimuli, such as injury. The SCs are considered bona fide adult stem cells in that they self-renew to maintain the SC pool and are able to create myogenic tissue, that is, muscle progenitors, throughout the lifespan. (C) Peak activation of various cell types for repair: various nonmyogenic and myogenic cells act in a coordinated fashion to ensure appropriate repair. Abbreviation: SC, satellite cell.
Importantly, the classic process of repair also requires many other nonmyogenic cellular players apart from the SC, all of which act in a concerted temporal fashion to ensure appropriate healing. During this process, the inflammatory cells, fibroblasts, endothelial cells, fibroadipogenic cells, and SCs interact with each other in a time-sensitive fashion (FIGURE 2C).9 Broadly speaking, after an injury, the immune response is initiated by the regional presence of monocytes and macrophages for clearing damaged tissue components. Following this initial response, there is a peak activation of the fibroblasts (1 week or less postinjury) and possibly the fibroadipogenic cells and the endothelial cells in order to create the ECM scaffold and vasculature. Finally, there is a marked increase in the numbers of SCs and myoblasts that repair the injured myofibers. In particular, the ECM-associated fibroblasts and the SCs have a complex relationship with each other. Once activated, the SCs facilitate remodeling of the local ECM by increased expression of matrix metallo-proteinases.90,132 The biomechanical and biochemical factors of the ECM also affect the functionality of the SC. For example, in vitro experiments have shown that the stiffness of the ECM affects not only the capacity of SCs to differentiate43 but also to self-renew.46 Apart from the ECM, quiescent SCs are present in close proximity to muscle microvasculature.17 During repair, the SC interactions with endothelial cells, that is, those responsible for new capillaries, are facilitated for the coordination of angiogenesis and myogenesis. It is important to understand that the total process of healing to return to a preinjury state can take up to 4 weeks,9 and is dependent on the severity of the injury. In many musculoskeletal conditions, like whiplash, where the injury and healing are not so simplistic, there can be maladaptation of this repair process and possibly functional outcomes on a patient-by-patient basis. It remains plausible that advanced imaging (eg, diffusion-weighted imaging and/or spectroscopy) could help identify acute-level changes in the muscle cell (or ECM) that could point to cause and effect and help to establish treatment planning/monitoring.
The expression of MFI has been well documented in injuries involving the rotator cuff complex. The injured rotator cuff muscles (supraspinatus and infraspinatus in particular) show abnormal fibrosis, fatty infiltration, and changes in fat deposits on CT, MRI, and ultrasound.66,77 These changes can be significant and may influence the outcome of the repair process.47 While it is not known how the specific changes occur at the cellular level, in humans the SC or skeletal muscle progenitors are higher in number in partial-thickness rotator cuff tears compared to noninjured and full-thickness tears.78 Importantly, these cells were unable to proliferate similarly, suggesting a decreased regenerative capacity and presumably a disruption of the dynamic balance between the cell types.
Interestingly, similar changes in muscle structure have been observed in animal injury models involving the lumbar spine. Specifically, rapid increases in adipocytes and connective tissue in the lumbar multifidus (porcine and ovine, respectively) have been observed following experimental lesions to both the intervertebral disc and nerve root.53,54 A follow-up study demonstrated that injury to the ovine intervertebral disc induces dramatic structural remodeling of the multifidus muscle, characterized by increased adipose and fibrotic tissue and muscle fiber changes, all of which provide for the exploration and development of novel interventions to impact muscle function and patient outcomes.54
While largely unknown, there is preliminary evidence that specific exercise can positively influence muscle structure and function in chronic whiplash.89 Further animal studies in which experimental lesions typical of a whiplash injury can be controlled for are required to better understand the repair process of skeletal muscle. Lastly, clinical trials investigating the parameters and effectiveness of various exercise programs targeting muscle structure and function are required before definitive conclusions can be drawn.
Key Points
Muscle stem cells, that is, SCs, play a key role in repair and regeneration after injury to muscle.
A number of other cell types, such as inflammatory cells, endothelial cells, fibroblasts, and fibroadipogenic cells, work in conjunction with SCs to ensure appropriate repair.
Maladaptation of this process could lead to pathological healing, such as excess connective tissue and fatty infiltration.
Future work is required to test the influence that specific exercise may have on the regeneration process.
ADVANCEMENTS IN NEUROIMAGING
Functional MRI
Functional MRI provides a means to indirectly measure neural activity by generating images that are sensitive to blood oxygenation levels (ie, blood oxygenation level-dependent [BOLD] contrast images).87,88 As neural activity increases in a brain region, blood flow to the activated region increases to deliver oxygen to meet the increased metabolic demands of the active neurons. The hemodynamic response during neural activity results in an uncoupling between blood flow and oxygen metabolism, such that blood flow increases more than the metabolic consumption of oxygen. This creates a local increase in the concentration of oxygenated hemoglobin and a local increase in signal on BOLD contrast images. In an fMRI study, the BOLD contrast images are collected over time to generate a series of images that can be statistically analyzed, and voxels that significantly fit the model are considered to be active.131
Functional MRI has been used extensively to map out the brain areas underlying the processing of nociceptive signals and the perception of pain in the brain (for review, see Apkarian et al5). Early fMRI pain studies primarily used experimental acute pain paradigms, in which painful stimuli were delivered over the course of the fMRI study (eg, with the participant in the scanner). While providing much information on the neural circuitry involved in acute pain, experimental pain is fundamentally different from the clinical pain experience, which is unique and personal to the patient experiencing the pain. A major advancement in neuroimaging has been the shift from experimental pain paradigms to the study of the brain regions underlying clinical pain.10,98,103 This work has demonstrated distinct differences in the patterns of brain activity for acute versus chronic pain conditions, with a prominent feature being that chronic pain appears to engage more of the brain’s emotional learning circuitry, suggesting a greater emotional component to chronic pain.6
The noninvasiveness of fMRI makes it an ideal tool for longitudinal studies and tracking the changes in the brain during the transition from acute to chronic pain. Baliki et al7 imaged patients over a course of a year following an initial episode of acute back pain. The strength of initial functional connectivity between the nucleus accumbens and prefrontal cortex was shown to be predictive of chronic back pain, implying that corticostriatal circuitry is causally involved in the transition from acute to chronic pain.7 A follow-up study demonstrated significant temporal reorganization of the connectivity between the hippocampus and the cingulate gyrus and medial prefrontal cortex.82 Specifically, the connectivity between the hippocampus and these brain areas decreased in those who continued to have pain compared to those who reported recovery, and the decrease in connectivity was correlated to the extent of the pain experienced 1 year later.82 These findings suggest that reorganization in the brain’s emotional learning circuitry may underlie the transition from acute to chronic pain, which has led to the theory that chronic pain results from a maladaptive learning process that converts the sensory-nociceptive-pain experiences following an initial injury to a pathological emotional state.4 This would certainly help to explain the expectation of recovery, maladaptive beliefs/cognition, stress system dysregulation, and perceived injustice following whiplash.
It is thus plausible that the neural circuitry involved in the chronicity of WAD partially parallels, or is similar to, that documented in chronic low back pain. In WAD, the MVC could be regarded as the initial painful event (time point zero), and, based on the state of the individual’s brain at time point zero, a prognostic “picture” could ensue. However, the application of fMRI and other neuroimaging techniques to specifically study WAD, unlike low back pain, has been limited to date. Linnman and colleagues,69 using positron emission tomography, found bilateral increases in regional cerebral blood flow in the posterior cingulate cortex and parahippocampal gyrus in individuals with WAD compared to pain-free controls.69 Such findings suggest that alterations in the brain’s resting state may be linked to an increased self-relevant evaluation of WAD-related pain and stress.69
An emerging and exciting area of research related to WAD has been the application of fMRI to study spinal cord function.126 The same principles underlying the BOLD signal in the brain should also apply to the spinal cord; however, spinal cord fMRI has been slower to develop due to technical issues with the imaging of the spinal cord and the analysis of spinal cord fMRI images.119 It is thus not surprising that the bulk of studies have been mainly proof-of-concept in nature, testing the feasibility of using spinal cord fMRI to detect signal changes during motor and sensory tasks.127 Recent advancements are beginning to overcome some of the mentioned technical barriers, and the validity and reliability of spinal cord fMRI are starting to be realized.126 With continued development, spinal cord fMRI may prove to be a valuable method to noninvasively study sensory and motor (FIGURE 3) processing in the spinal cord and the changes in spinal cord function following injury to peripheral structures or the direct injury of the spinal cord.16,65
FIGURE 3.
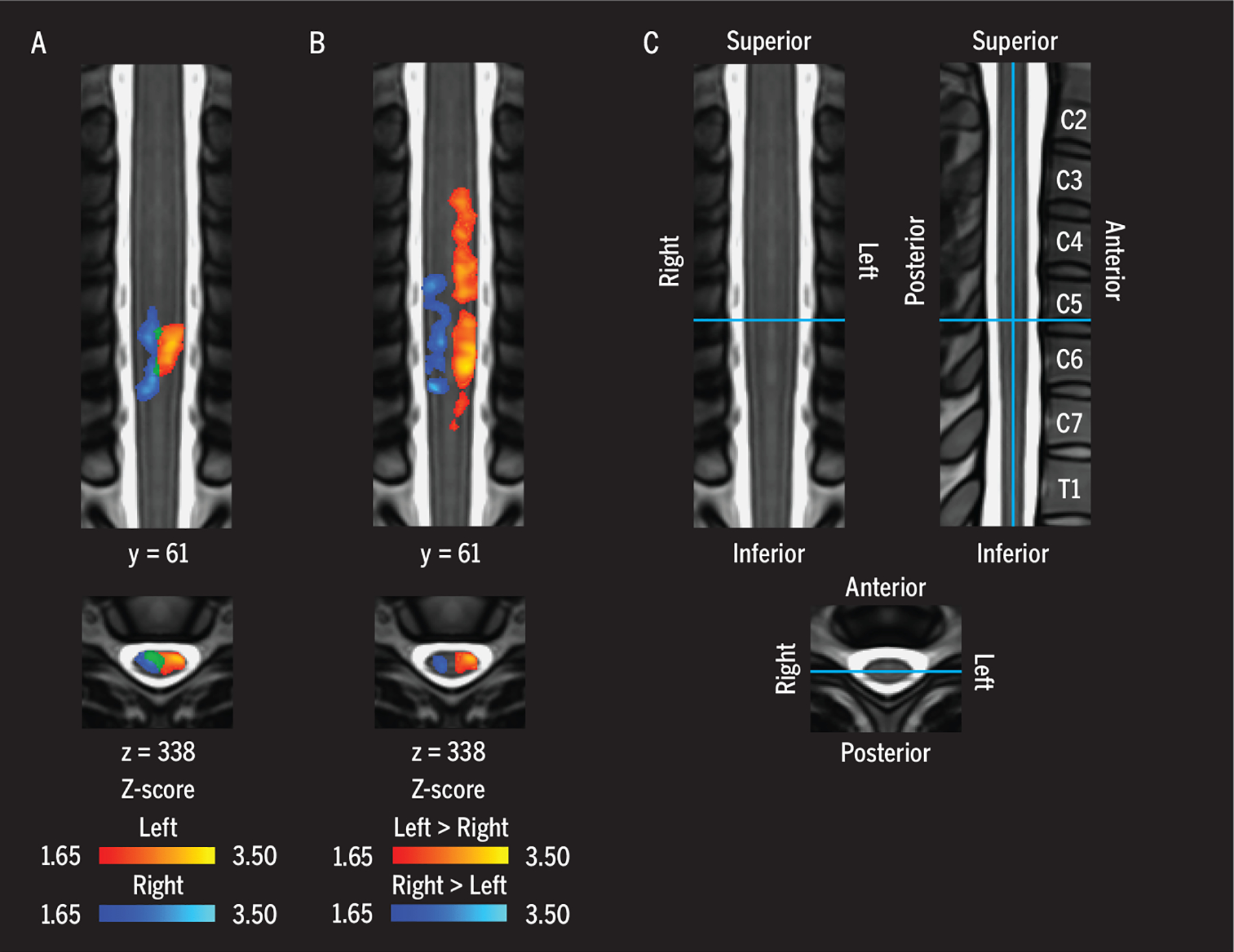
(A) Functional images demonstrating group average cervical spinal cord activity during a left-(red-yellow) and right-sided (blue-light blue) isometric upper extremity motor task. (B) Areas where the signal was greater for the left-sided task compared to the right-sided task, and vice versa. The activity was lateralized to the hemicord ipsilateral to the side of the task. (C) The legend shows the location of the coronal and axial slices on the MNI-Poly-AMU spinal cord template, with corresponding vertebrae labeled. Adapted with permission from Weber et al.126 Copyright ©2016 Elsevier.
Overall, greater application of fMRI to study the potential reorganization of the brain and spinal cord in individuals with WAD is warranted. Longitudinal studies are needed to expand on the work that has been performed in low back pain. Further use of fMRI in these individuals may lead to the discovery and development of patient-specific multimodal treatment pathways that incorporate cognitive behavioral and neural feedback techniques in tandem with physical therapy.
Key Points
Functional MRI provides a means to visualize indirect patterns of neural activity in the human brain and spinal cord.
Neuroimaging has revealed differences in neural circuitry in individuals with acute and chronic low back pain; however, this has yet to be sufficiently studied in individuals with traumatic neck pain.
MAGNETIZATION TRANSFER IMAGING
Magnetization transfer (MT) is an MRI method that creates contrast based on the macromolecular content of tissues (FIGURE 4).52 In addition, by comparing images with and without MT contrast (known as MT ratio), the MT effect can be used to explore and quantify anomalies in brain white matter across multiple neurologic pathologies (eg, multiple sclerosis109 and traumatic brain injury110). Magnetization transfer has also been shown to be sensitive to spinal cord demyelination.19
FIGURE 4.
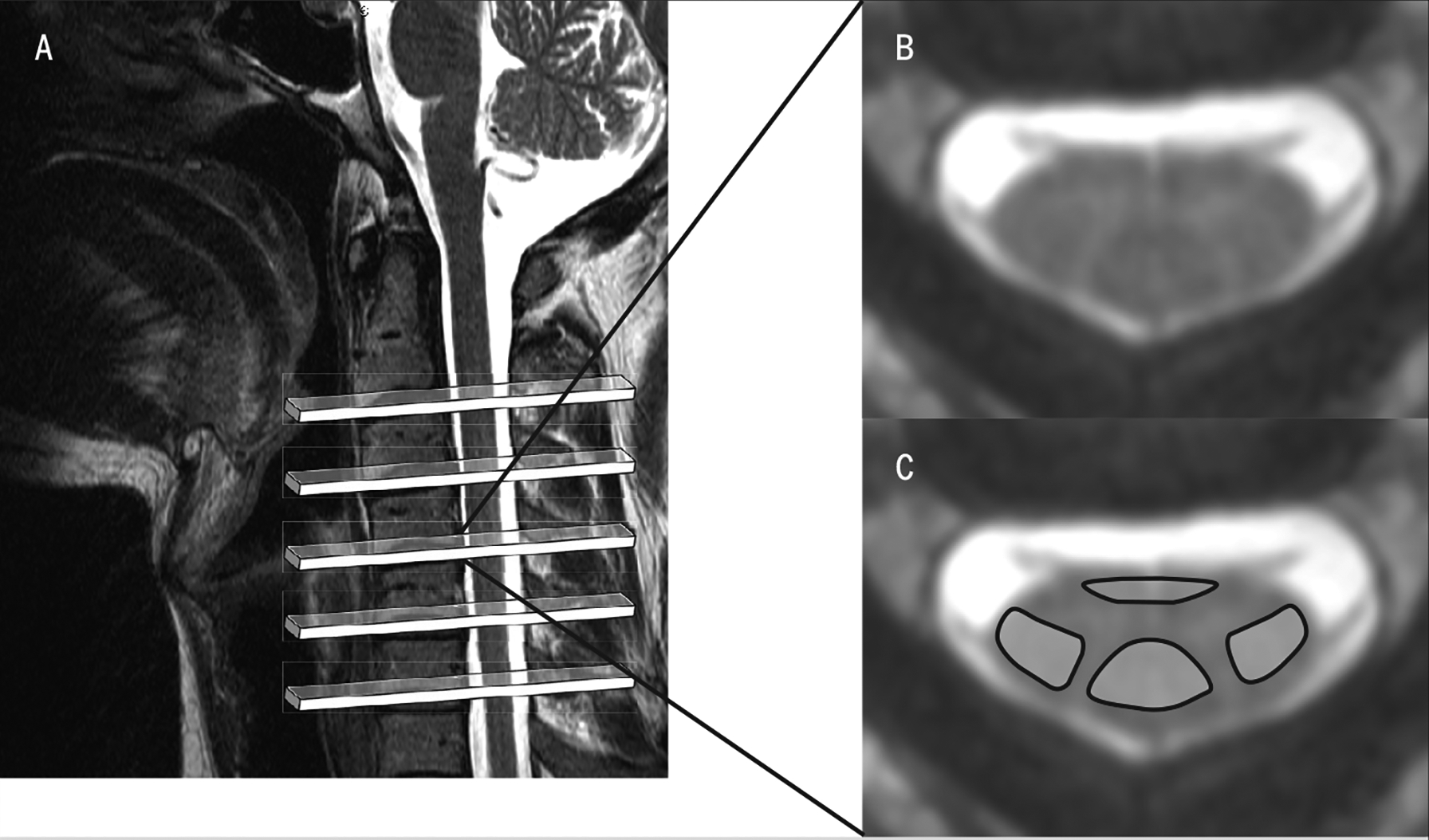
(A) A T2-weighted magnetic resonance image was used to plan the axial slices for MT- and non—MT-weighted acquisition, spanning the C2–7 area of the spinal cord. (B) Magnetization transfer image corresponding to the region caudal to C4. (C) Non-MT image in the same region, with anatomically defined regions of interest over the ventromedial and dorsal columns of the spinal cord. These images are used to measure the MT ratio, quantifying anomalies in white-matter pathways. Abbreviation: MT, magnetization transfer.
In considering the pathomechanics of the typical whiplash injury event, a potential underlying mechanism, at least in a subset of patients, could involve a mild insult to the spinal cord. Conversely, the lack of typical radiological signs of an injured cord (eg, edema or hemorrhage on T2-weighted MRI in known spinal cord injury14) would suggest this is not the case. A recent, albeit preliminary, case series using MT imaging has quantified the presence of losses in spinal cord motor pathways, without the characteristic signal from edema/hemorrhage, in a small sample of patients with chronic WAD.36 Implementation of high-field MT measures of the cervical spinal cord in the acute stage could potentially complement the objective assessment and projection of functional recovery.102
SPECTROSCOPY
For nearly 80 years, scientists have used magnetic resonance spectroscopy (MRS) to determine chemical structure and function, and understand the underlying mechanisms of various disease states. While the majority of radiographic investigations into the pathophysiology of WAD (and other conditions such as myelopathy57) have focused on damage/injury to anatomy on a macroscopic scale, spectroscopy can be used to observe and quantify biochemical function/dysfunction (eg, altered metabolites) of the brain, spinal cord, and skeletal muscle on a microscopic scale.
In proton MRS, the common visible metabolites are creatine, choline, lactate, lipid peaks, N-acetylacetate, and citrate. Phosphorus spectroscopy is used to visualize metabolites such as adenosine triphosphate, phosphocreatine, phosphomonoesters, phosphodiesters, and inorganic phosphate. As the strength of the magnetic field increases, the separation between peaks and signal to noise with MRS improves, providing a more robust environment in which to noninvasively evaluate cellular biochemistry following head/neck trauma—and possibly add to the prediction of recovery. There is evidence from MRS-based studies toward the prediction of neurologic recovery from pediatric trauma (“shaken-baby” syndrome)51 and the identification of biomarkers in spondylosis.107 Preliminary evidence from a small cohort of individuals with persistent whiplash-associated pain suggests that biochemical changes in the central nervous system could be present.40 To our knowledge, the usefulness of MRS has yet to be investigated in the acute phase of whiplash or in muscles of individuals with WAD. However, the acute phase may be an optimal time to consider MRS of muscle tissue, as the expected lack of MFI would prevent any macroscopic lipid contamination.
DIFFUSION-WEIGHTED IMAGING
Given that rapid compositional changes in muscles either directly or indirectly impacted by trauma may help predict of the rate and extent of recovery following MVC, techniques with the sensitivity to detect abnormalities during the first hours or days postinjury hold considerable promise as clinical tools. One such technique may be DW-MRI, which is already used widely in the clinical setting to identify regions of cerebral ischemia associated with neurological insults (eg, stroke). Indeed, DW-MRI can reveal regions of restricted blood flow well in advance of conventional imaging approaches (eg, T2-weighted MRI, CT).111 In DW-MRI, signal intensity is directly related to the rate of water diffusion through a given tissue.76 Diffusion-weighted MRI scans are acquired at progressively increasing levels of diffusion weighting, and from these images a composite image is generated that represents the apparent diffusion coefficient (ADC) at each voxel. This so-called ADC map enables visualization of complex tissue properties, and can be used to highlight contrast between regions with relatively free or restricted flow.
Diffusion-weighted MRI can be applied to skeletal muscle,31,134 where it has been used to detect both temporary exercise-induced changes in regional blood flow81,135 and early signs of denervation56 (FIGURE 5). For example, significant increases in muscle ADC can be observed during and following brief submaximal isometric contractions,133 following peripheral nerve injury and associated denervation,48 and in individuals with subacute lumbosacral radiculopathy.56 Although changes in ADC following denervation appear to precede findings from electrophysiological testing,56 it remains unknown how the relative magnitude and time course of ADC changes relate to other pathophysiological muscle biomarkers (eg, MFI and potential MRS-related findings). Given the relatively recent application of DW-MRI to skeletal muscle, systematic investigation of short and long-term reliability is required prior to any potential clinical consideration of the evaluation of a patient following whiplash in whom denervation or disuse atrophy of the neck or extremity muscles may be suspected. Nevertheless, DW-MRI (potentially in conjunction with MRS) may provide critical early insights into the progression of WAD.
FIGURE 5.
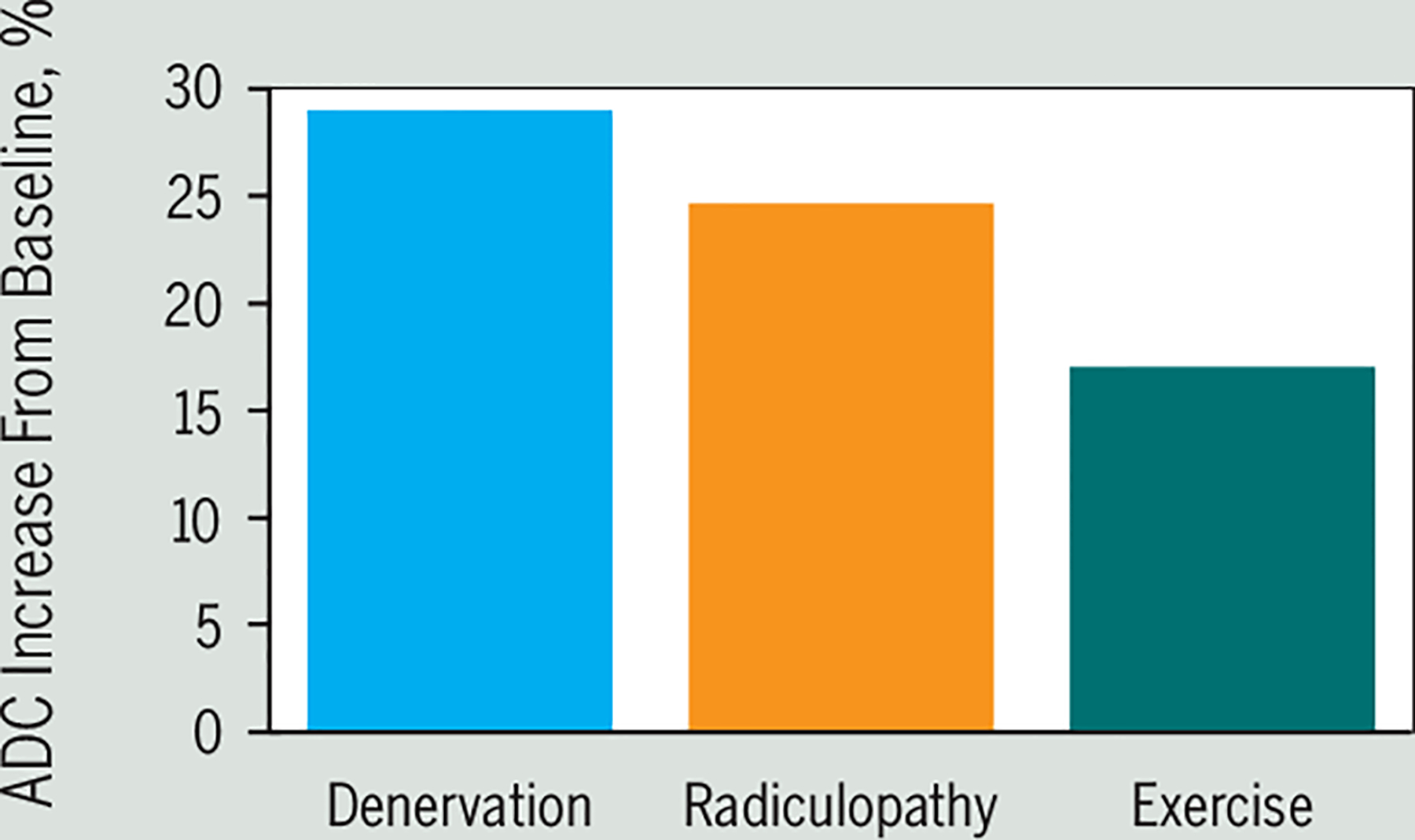
Changes in muscle physiology lead to increased ADC. Complete denervation of the hamstring (blue bar) leads to a rapid (1 day after injury) increase (approximately 30%) in ADC. An approximate 25% increase in ADC has been observed within 1 month following onset of lumbosacral radiculopathy (orange bar), and brief submaximal contractions (50% maximum volitional contraction) can lead to approximately 15% to 20% increases in ADC after 10 minutes postexercise (green bar). Abbreviation: ADC, apparent diffusion coefficient. Denervation and radiculopathy data adapted from Holl et al,56 and exercise data adapted from Yanagisawa et al.135
Key Points
High-resolution MT sequences could be a means of further characterizing the whiplash condition by identifying early signs of changes in spinal cord or brain functioning.
Magnetic resonance spectroscopy of the brain and spinal cord could provide a potentially clinically useful biomarker for the characterization and management of enigmatic musculoskeletal conditions, and, if adapted to skeletal muscle, may be particularly viable in the acute phase of WAD prior to any changes in muscle structure.
Early alterations in water diffusion within skeletal muscles, visualized with diffusion-weighted imaging, may be associated with the clinical course. Thus, DW-MRI may be a useful imaging application to assess physiologic variations in the acute stage.
SUMMARY
The Imaging Measures And Mechanistic models described in this paper represent a number of advances toward explaining the genesis of chronic posttraumatic problems (with or without concern for significant pathology), owing to the pioneering work of many clinician/research scientists before us. The intention of ongoing work in this field is to grow from what we have learned, building off a platform on which biology, psychology, and sociology can be integrated to further our understanding of why some but not others transition from acute to chronic pain-related disability. Readers should be aware that while current mechanistic models and measures represent new and exciting starting points, they are not to be considered definitive end points. With time and new research findings, it is anticipated that some of these models and measures will be modified, combined, expanded, or refined.
Patients with chronic WAD experience a multidimensional condition, with numerous medical and noninjury-related factors influencing outcomes.26 Similarly, clinicians and investigators are challenged to understand and approach care of patients with this condition from a commensurate number of perspectives. While not exhaustive, the advancing imaging measures described within this paper offer a glimpse of the largely untapped potential for mechanistic cross-disciplinary research to have an impact on a condition that is both common and costly and can lead to considerable long term pain and disability for some.
Acknowledgments
Dr Elliott is funded by the National Institutes of Health (NICHD/NCMRR grant 1 R01 HD079076–01A1). Dr Elliott’s relevant financial activities outside the body of work include a 35% ownership/investment in a medical consulting start-up, Pain ID, LLC. The authors certify that they have no affiliations with or financial involvement in any organization or entity with a direct financial interest in the subject matter or materials discussed in the article.
APPENDIX
IMAGING CONDITIONS CITED IN SUSPECTED SPINE TRAUMA (AMERICAN COLLEGE OF RADIOLOGY APPROPRIATENESS CRITERIA22)
Canadian Cervical Spine Rule18,117,118.
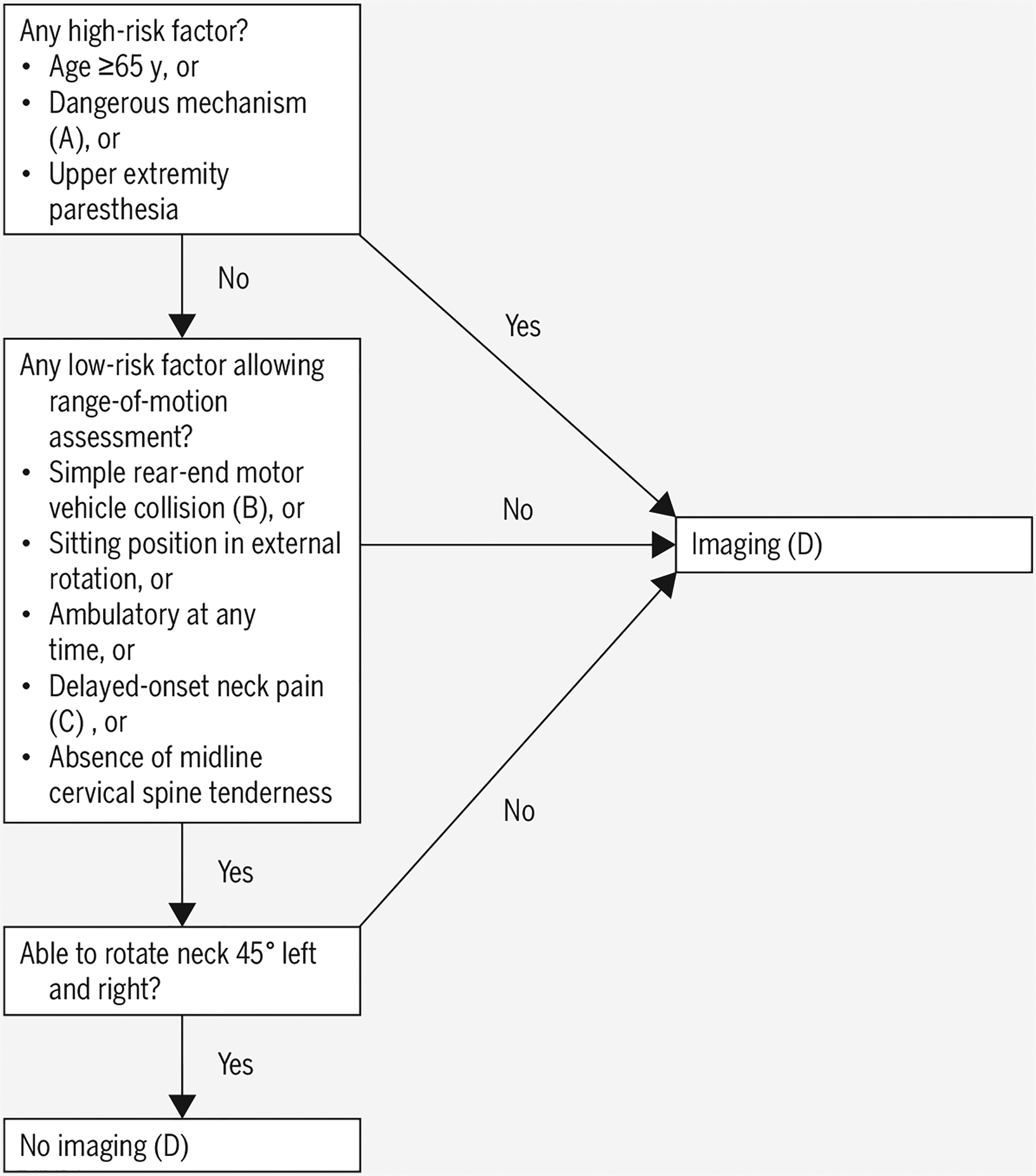
A. Dangerous mechanism: fall from 3 ft/5 stairs or higher, axial load, motor vehicle collision at greater than 60 mph or rollover or ejection, motorized recreational vehicle accident, bicycle collision
B. Simple rear-end motor vehicle collision excludes: pushed into oncoming traffic, hit by bus or large truck, rollover, hit by high-speed vehicle
C. Delayed-onset neck pain: no immediate onset after trauma
D. At time of derivation, radiograph was chosen imaging. Now, American College of Radiology recommends computed tomography if positive on criteria
E. At time of derivation, radiograph was chosen imaging. Now, American College of Radiology recommends computed tomography if positive on criteria
National Emergency X-Radiography Utilization Study Low-Risk Rule55,91
Low probability of cervical spine injury if all 5 met:
No tenderness at posterior midline of the cervical spine
No focal neurological deficit
Normal level of alertness
No evidence of intoxication
No clinically apparent painful injury that might distract from pain of a cervical spine injury
REFERENCES
- 1.American College of Radiology. ACR Appropriateness Criteria: chronic neck pain. Available at: https://acsearch.acr.org/docs/69426/Narrative/. Accessed December 21, 2015. [PubMed]
- 2.American College of Radiology. ACR Appropriateness Criteria: low back pain. Available at: https://acsearch.acr.org/docs/69483/Narrative/. Accessed August 22, 2016.
- 3.Anderson SE, Boesch C, Zimmermann H, et al. Are there cervical spine findings at MR imaging that are specific to acute symptomatic whiplash injury? A prospective controlled study with four experienced blinded readers. Radiology. 2012;262:567–575. 10.1148/radiol.11102115 [DOI] [PubMed] [Google Scholar]
- 4.Apkarian AV, Baliki MN, Farmer MA. Predicting transition to chronic pain. Curr Opin Neurol. 2013;26:360–367. 10.1097/WCO.0b013e32836336ad [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Apkarian AV, Bushnell MC, Treede RD, Zubieta JK. Human brain mechanisms of pain perception and regulation in health and disease. Eur J Pain. 2005;9:463–484. 10.1016/j.ejpain.2004.11.001 [DOI] [PubMed] [Google Scholar]
- 6.Apkarian AV, Hashmi JA, Baliki MN. Pain and the brain: specificity and plasticity of the brain in clinical chronic pain. Pain. 2011;152:S49–S64. 10.1016/j.pain.2010.11.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baliki MN, Petre B, Torbey S, et al. Corticostriatal functional connectivity predicts transition to chronic back pain. Nat Neurosci. 2012;15:1117–1119. 10.1038/nn.3153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bayless P, Ray VG. Incidence of cervical spine injuries in association with blunt head trauma. Am J Emerg Med. 1989;7:139–142. [DOI] [PubMed] [Google Scholar]
- 9.Bentzinger CF, Wang YX, Dumont NA, Rudnicki MA. Cellular dynamics in the muscle satellite cell niche. EMBO Rep. 2013;14:1062–1072. 10.1038/embor.2013.182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bingel U, Lorenz J, Schoell E, Weiller C, Büchel C. Mechanisms of placebo analgesia: rACC recruitment of a subcortical antinociceptive network. Pain. 2006;120:8–15. 10.1016/j.pain.2005.08.027 [DOI] [PubMed] [Google Scholar]
- 11.Blum CA, Yaghi S. Cervical artery dissection: a review of the epidemiology, pathophysiology, treatment, and outcome. Arch Neurosci. 2015;2:e26670 10.5812/archneurosci.26670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bortsov AV, Diatchenko L, McLean SA. Complex multilocus effects of catechol-O-methyltransferase haplotypes predict pain and pain interference 6 weeks after motor vehicle collision. Neuromolecular Med. 2014;16:83–93. 10.1007/s12017-013-8255-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bortsov AV, Smith JE, Diatchenko L, et al. Polymorphisms in the glucocorticoid receptor co-chaperone FKBP5 predict persistent musculoskeletal pain after traumatic stress exposure. Pain. 2013;154:1419–1426. 10.1016/j.pain.2013.04.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bozzo A, Marcoux J, Radhakrishna M, Pelletier J, Goulet B. The role of magnetic resonance imaging in the management of acute spinal cord injury. J Neurotrauma. 2011;28:1401–1411. 10.1089/neu.2009.1236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carroll LJ, Holm LW, Hogg-Johnson S, et al. Course and prognostic factors for neck pain in whiplash-associated disorders (WAD): results of the Bone and Joint Decade 2000–2010 Task Force on Neck Pain and Its Associated Disorders. Spine (Phila Pa 1976). 2008;33:S83–S92. 10.1097/BRS.0b013e3181643eb8 [DOI] [PubMed] [Google Scholar]
- 16.Chen LM, Mishra A, Yang PF, Wang F, Gore JC. Injury alters intrinsic functional connectivity within the primate spinal cord. Proc Natl Acad Sci U S A. 2015;112:5991–5996. 10.1073/pnas.1424106112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Christov C, Chrétien F, Abou-Khalil R, et al. Muscle satellite cells and endothelial cells: close neighbors and privileged partners. Mol Biol Cell. 2007;18:1397–1409. 10.1091/mbc.E06-08-0693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Coffey F, Hewitt S, Stiell I, et al. Validation of the Canadian C-Spine Rule in the UK emergency department setting. Emerg Med J. 2011;28:873–876. 10.1136/emj.2009.089508 [DOI] [PubMed] [Google Scholar]
- 19.Cohen-Adad J, El Mendili MM, Lehéricy S, et al. Demyelination and degeneration in the injured human spinal cord detected with diffusion and magnetization transfer MRI. Neuroimage. 2011;55:1024–1033. 10.1016/j.neuroimage.2010.11.089 [DOI] [PubMed] [Google Scholar]
- 20.Collins CA, Olsen I, Zammit PS, et al. Stem cell function, self-renewal, and behavioral heterogeneity of cells from the adult muscle satellite cell niche. Cell. 2005;122:289–301. 10.1016/j.cell.2005.05.010 [DOI] [PubMed] [Google Scholar]
- 21.Curatolo M, Bogduk N, Ivancic PC, McLean SA, Siegmund GP, Winkelstein BA. The role of tissue damage in whiplash-associated disorders: discussion paper 1. Spine (Phila Pa 1976). 2011;36:S309–S315. 10.1097/BRS.0b013e318238842a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Daffner RH, Hackney DB. ACR Appropriateness Criteria® on suspected spine trauma. J Am Coll Radiol. 2007;4:762–775. 10.1016/j.jacr.2007.08.006 [DOI] [PubMed] [Google Scholar]
- 23.Dayanidhi S, Lieber RL. Skeletal muscle satellite cells: mediators of muscle growth during development and implications for developmental disorders. Muscle Nerve. 2014;50:723–732. 10.1002/mus.24441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Debette S. Pathophysiology and risk factors of cervical artery dissection: what have we learnt from large hospital-based cohorts? Curr Opin Neurol. 2014;27:20–28. 10.1097/WCO.0000000000000056 [DOI] [PubMed] [Google Scholar]
- 25.Desai NK, Kang J, Chokshi FH. Screening CT angiography for pediatric blunt cerebrovascular injury with emphasis on the cervical “seatbelt sign”. AJNR Am J Neuroradiol. 2014;35:1836–1840. 10.3174/ajnr.A3916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dufton JA, Bruni SG, Kopec JA, Cassidy JD, Quon J. Delayed recovery in patients with whiplash-associated disorders. Injury. 2012;43:1141–1147. 10.1016/j.injury.2012.03.006 [DOI] [PubMed] [Google Scholar]
- 27.Dulor JP, Cambon B, Vigneron P, et al. Expression of specific white adipose tissue genes in denervation-induced skeletal muscle fatty degeneration. FEBS Lett. 1998;439:89–92. 10.1016/S0014-5793(98)01216-2 [DOI] [PubMed] [Google Scholar]
- 28.Elliott J, Cannata E, Christensen E, et al. MRI analysis of the size and shape of the oropharynx in chronic whiplash. Otolaryngol Head Neck Surg. 2008;138:747–751. 10.1016/j.otohns.2008.02.015 [DOI] [PubMed] [Google Scholar]
- 29.Elliott J, Jull G, Noteboom JT, Darnell R, Galloway G, Gibbon WW. Fatty infiltration in the cervical extensor muscles in persistent whiplash-associated disorders: a magnetic resonance imaging analysis. Spine (Phila Pa 1976). 2006;31:E847–E855. 10.1097/01.brs.0000240841.07050.34 [DOI] [PubMed] [Google Scholar]
- 30.Elliott J, Jull G, Noteboom JT, Galloway G. MRI study of the cross-sectional area for the cervical extensor musculature in patients with persistent whiplash associated disorders (WAD). Man Ther. 2008;13:258–265. 10.1016/j.math.2007.01.012 [DOI] [PubMed] [Google Scholar]
- 31.Elliott J, Pedler A, Beattie P, McMahon K. Diffusion-weighted magnetic resonance imaging for the healthy cervical multifidus: a potential method for studying neck muscle physiology following spinal trauma. J Orthop Sports Phys Ther. 2010;40:722–728. 10.2519/jospt.2010.3423 [DOI] [PubMed] [Google Scholar]
- 32.Elliott J, Pedler A, Kenardy J, Galloway G, Jull G, Sterling M. The temporal development of fatty infiltrates in the neck muscles following whiplash injury: an association with pain and posttraumatic stress. PLoS One. 2011;6:e21194 10.1371/journal.pone.0021194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Elliott J, Sterling M, Noteboom JT, Darnell R, Galloway G, Jull G. Fatty infiltrate in the cervical extensor muscles is not a feature of chronic, insidious-onset neck pain. Clin Radiol. 2008;63:681–687. 10.1016/j.crad.2007.11.011 [DOI] [PubMed] [Google Scholar]
- 34.Elliott JM. Are there implications for morphological changes in neck muscles after whiplash injury? Spine (Phila Pa 1976). 2011;36:S205–S210. 10.1097/BRS.0b013e3182387f57 [DOI] [PubMed] [Google Scholar]
- 35.Elliott JM, Courtney DM, Rademaker A, Pinto D, Sterling MM, Parrish TB. The rapid and progressive degeneration of the cervical multifidus in whiplash: an MRI study of fatty infiltration. Spine (Phila Pa 1976). 2015;40:E694–E700. 10.1097/BRS.0000000000000891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Elliott JM, Dewald JP, Hornby TG, Walton DM, Parrish TB. Mechanisms underlying chronic whiplash: contributions from an incomplete spinal cord injury? Pain Med. 2014;15:1938–1944. 10.1111/pme.12518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Elliott JM, Kerry R, Flynn T, Parrish TB. Content not quantity is a better measure of muscle degeneration in whiplash. Man Ther. 2013;18:578–582. 10.1016/j.math.2013.02.002 [DOI] [PubMed] [Google Scholar]
- 38.Elliott JM, Noteboom JT, Flynn TW, Sterling M. Characterization of acute and chronic whiplash-associated disorders. J Orthop Sports Phys Ther. 2009;39:312–323. 10.2519/jospt.2009.2826 [DOI] [PubMed] [Google Scholar]
- 39.Elliott JM, O’Leary S, Sterling M, Hendrikz J, Pedler A, Jull G. Magnetic resonance imaging findings of fatty infiltrate in the cervical flexors in chronic whiplash. Spine (Phila Pa 1976). 2010;35:948–954. 10.1097/BRS.0b013e3181bb0e55 [DOI] [PubMed] [Google Scholar]
- 40.Elliott JM, Pedler AR, Cowin G, Sterling M, McMahon K. Spinal cord metabolism and muscle water diffusion in whiplash. Spinal Cord. 2012;50:474–476. 10.1038/sc.2011.17 [DOI] [PubMed] [Google Scholar]
- 41.Elliott JM, Pedler AR, Jull GA, Van Wyk L, Galloway GG, O’Leary SP. Differential changes in muscle composition exist in traumatic and nontraumatic neck pain. Spine (Phila Pa 1976). 2014;39:39–47. 10.1097/BRS.0000000000000033 [DOI] [PubMed] [Google Scholar]
- 42.Elliott JM, Pedler AR, Theodoros D, Jull GA. Magnetic resonance imaging changes in the size and shape of the oropharynx following acute whiplash injury. J Orthop Sports Phys Ther. 2012;42:912–918. 10.2519/jospt.2012.4280 [DOI] [PubMed] [Google Scholar]
- 43.Engler AJ, Griffin MA, Sen S, Bönnemann CG, Sweeney HL, Discher DE. Myotubes differentiate optimally on substrates with tissue-like stiffness: pathological implications for soft or stiff microenvironments. J Cell Biol. 2004;166:877–887. 10.1083/jcb.200405004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fischer RP. Cervical radiographic evaluation of alert patients following blunt trauma. Ann Emerg Med. 1984;13:905–907. 10.1016/S0196-0644(84)80667-8 [DOI] [PubMed] [Google Scholar]
- 45.Gbaanador GB, Fruin AH, Taylon C. Role of routine emergency cervical radiography in head trauma. Am J Surg. 1986;152:643–648. 10.1016/0002-9610(86)90441-1 [DOI] [PubMed] [Google Scholar]
- 46.Gilbert PM, Havenstrite KL, Magnusson KE, et al. Substrate elasticity regulates skeletal muscle stem cell self-renewal in culture. Science. 2010;329:1078–1081. 10.1126/science.1191035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gladstone JN, Bishop JY, Lo IK, Flatow EL. Fatty infiltration and atrophy of the rotator cuff do not improve after rotator cuff repair and correlate with poor functional outcome. Am J Sports Med. 2007;35:719–728. 10.1177/0363546506297539 [DOI] [PubMed] [Google Scholar]
- 48.Goyault G, Bierry G, Holl N, et al. Diffusion-weighted MRI, dynamic susceptibility contrast MRI and ultrasound perfusion quantification of denervated muscle in rabbits. Skeletal Radiol. 2012;41:33–40. 10.1007/s00256-011-1108-4 [DOI] [PubMed] [Google Scholar]
- 49.Grauer JN, Panjabi MM, Cholewicki J, Nibu K, Dvorak J. Whiplash produces an S-shaped curvature of the neck with hyperextension at lower levels. Spine (Phila Pa 1976). 1997;22:2489–2494. [DOI] [PubMed] [Google Scholar]
- 50.Harrigan MR, Hadley MN, Dhall SS, et al. Management of vertebral artery injuries following non-penetrating cervical trauma. Neurosurgery. 2013;72 suppl 2:234–243. 10.1227/NEU.0b013e31827765f5 [DOI] [PubMed] [Google Scholar]
- 51.Haseler LJ, Arcinue E, Danielsen ER, Bluml S, Ross BD. Evidence from proton magnetic resonance spectroscopy for a metabolic cascade of neuronal damage in shaken baby syndrome. Pediatrics. 1997;99:4–14. [DOI] [PubMed] [Google Scholar]
- 52.Henkelman RM, Stanisz GJ, Graham SJ. Magnetization transfer in MRI: a review. NMR Biomed. 2001;14:57–64. 10.1002/nbm.683 [DOI] [PubMed] [Google Scholar]
- 53.Hodges P, Holm AK, Hansson T, Holm S. Rapid atrophy of the lumbar multifidus follows experimental disc or nerve root injury. Spine (Phila Pa 1976). 2006;31:2926–2933. 10.1097/01.brs.0000248453.51165.0b [DOI] [PubMed] [Google Scholar]
- 54.Hodges PW, James G, Blomster L, et al. Multifidus muscle changes after back injury are characterized by structural remodeling of muscle, adipose and connective tissue, but not muscle atrophy: molecular and morphological evidence. Spine (Phila Pa 1976). 2015;40:1057–1071. 10.1097/BRS.0000000000000972 [DOI] [PubMed] [Google Scholar]
- 55.Hoffman JR, Wolfson AB, Todd K, Mower WR. Selective cervical spine radiography in blunt trauma: methodology of the National Emergency X-Radiography Utilization Study (NEXUS). Ann Emerg Med. 1998;32:461–469. 10.1016/S0196-0644(98)70176-3 [DOI] [PubMed] [Google Scholar]
- 56.Holl N, Echaniz-Laguna A, Bierry G, et al. Diffusion-weighted MRI of denervated muscle: a clinical and experimental study. Skeletal Radiol. 2008;37:1111–1117. 10.1007/s00256-008-0552-2 [DOI] [PubMed] [Google Scholar]
- 57.Holly LT, Freitas B, McArthur DL, Salamon N. Proton magnetic resonance spectroscopy to evaluate spinal cord axonal injury in cervical spondylotic myelopathy. J Neurosurg Spine. 2009;10:194–200. 10.3171/2008.12.SPINE08367 [DOI] [PubMed] [Google Scholar]
- 58.Ivancic PC, Ito S, Tominaga Y, Carlson EJ, Rubin W, Panjabi MM. Effect of rotated head posture on dynamic vertebral artery elongation during simulated rear impact. Clin Biomech (Bristol, Avon). 2006;21:213–220. 10.1016/j.clinbiomech.2005.10.011 [DOI] [PubMed] [Google Scholar]
- 59.Ivancic PC, Xiao M. Cervical spine curvature during simulated rear crashes with energy-absorbing seat. Spine J. 2011;11:224–233. 10.1016/j.spinee.2011.01.025 [DOI] [PubMed] [Google Scholar]
- 60.Ivancic PC, Xiao M. Understanding whiplash injury and prevention mechanisms using a human model of the neck. Accid Anal Prev. 2011;43:1392–1399. 10.1016/j.aap.2011.02.014 [DOI] [PubMed] [Google Scholar]
- 61.Jull G, Kenardy J, Hendrikz J, Cohen M,Sterling M. Management of acute whiplash: a randomized controlled trial of multidisciplinary stratified treatments. Pain. 2013;154:1798–1806. 10.1016/j.pain.2013.05.041 [DOI] [PubMed] [Google Scholar]
- 62.Kaneoka K, Ono K, Inami S, Hayashi K. Motion analysis of cervical vertebrae during whiplash loading. Spine (Phila Pa 1976). 1999;24:763–769; discussion 770. [DOI] [PubMed] [Google Scholar]
- 63.Karlsson A, Leinhard OD, Åslund U, et al. An investigation of fat infiltration of the multifidus muscle in patients with severe neck symptoms associated with chronic whiplash-associated disorder. J Orthop Sports Phys Ther. 2016;46:886–893. 10.2519/jospt.2016.6553 [DOI] [PubMed] [Google Scholar]
- 64.Khan M, Chong ST, Mazza MB, Hoff C, Albeiruti AR. Clearing the posttraumatic adult cervical spine. Contemp Diagn Radiol. 2013;36:1–7. 10.1097/01.CDR.0000428991.36017.0b [DOI] [Google Scholar]
- 65.Kolesar TA, Fiest KM, Smith SD, Kornelsen J. Assessing nociception by fMRI of the human spinal cord: a systematic review. Magn Reson Insights. 2015;8:31–39. 10.4137/MRI.S23556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kuzel BR, Grindel S, Papandrea R, Ziegler D. Fatty infiltration and rotator cuff atrophy. J Am Acad Orthop Surg. 2013;21:613–623. 10.5435/JAAOS-21-10-613 [DOI] [PubMed] [Google Scholar]
- 67.Lamb SE, Gates S, Williams MA, et al. Emergency department treatments and physiotherapy for acute whiplash: a pragmatic, two-step, randomised controlled trial. Lancet. 2013;381:546–556. 10.1016/S0140-6736(12)61304-X [DOI] [PubMed] [Google Scholar]
- 68.Lepper C, Partridge TA, Fan CM. An absolute requirement for Pax7-positive satellite cells in acute injury-induced skeletal muscle regeneration. Development. 2011;138:3639–3646. 10.1242/dev.067595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Linnman C, Appel L, Söderlund A, et al. Chronic whiplash symptoms are related to altered regional cerebral blood flow in the resting state. Eur J Pain. 2009;13:65–70. 10.1016/j.ejpain.2008.03.001 [DOI] [PubMed] [Google Scholar]
- 70.Mathen R, Inaba K, Munera F, et al. Prospective evaluation of multislice computed tomography versus plain radiographic cervical spine clearance in trauma patients. J Trauma. 2007;62:1427–1431. 10.1097/01.ta.0000239813.78603.15 [DOI] [PubMed] [Google Scholar]
- 71.Matsumoto M, Ichihara D, Okada E, et al. Cross-sectional area of the posterior extensor muscles of the cervical spine in whiplash injury patients versus healthy volunteers – 10 year follow-up MR study. Injury. 2012;43:912–916. 10.1016/j.injury.2012.01.017 [DOI] [PubMed] [Google Scholar]
- 72.Matsumoto M, Okada E, Ichihara D, et al. Prospective ten-year follow-up study comparing patients with whiplash-associated disorders and asymptomatic subjects using magnetic resonance imaging. Spine (Phila Pa 1976). 2010;35:1684–1690. 10.1097/BRS.0b013e3181c9a8c7 [DOI] [PubMed] [Google Scholar]
- 73.McKay BR, Toth KG, Tarnopolsky MA, Parise G. Satellite cell number and cell cycle kinetics in response to acute myotrauma in humans: immunohistochemistry versus flow cytometry. J Physiol. 2010;588:3307–3320. 10.1113/jphysiol.2010.190876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.McLean SA. The potential contribution of stress systems to the transition to chronic whiplash-associated disorders. Spine (Phila Pa 1976). 2011;36:S226–S232. 10.1097/BRS.0b013e3182387fb4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.McLean SA, Clauw DJ, Abelson JL, Liberzon I. The development of persistent pain and psychological morbidity after motor vehicle collision: integrating the potential role of stress response systems into a biopsychosocial model. Psychosom Med. 2005;67:783–790. 10.1097/01.psy.0000181276.49204.bb [DOI] [PubMed] [Google Scholar]
- 76.McMahon KL, Cowin G, Galloway G. Magnetic resonance imaging: the underlying principles. J Orthop Sports Phys Ther. 2011;41:806–819. 10.2519/jospt.2011.3576 [DOI] [PubMed] [Google Scholar]
- 77.Melis B, DeFranco MJ, Chuinard C, Walch G. Natural history of fatty infiltration and atrophy of the supraspinatus muscle in rotator cuff tears. Clin Orthop Relat Res. 2010;468:1498–1505. 10.1007/s11999-009-1207-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Meyer GA, Farris AL, Sato E, et al. Muscle progenitor cell regenerative capacity in the torn rotator cuff. J Orthop Res. 2015;33:421–429. 10.1002/jor.22786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Michaleff ZA, Maher CG, Lin CW, et al. Comprehensive physiotherapy exercise programme or advice for chronic whiplash (PROMISE): a pragmatic randomised controlled trial. Lancet. 2014;384:133–141. 10.1016/S0140-6736(14)60457-8 [DOI] [PubMed] [Google Scholar]
- 80.Michaleff ZA, Maher CG, Verhagen AP, Rebbeck T, Lin CW. Accuracy of the Canadian C-spine rule and NEXUS to screen for clinically important cervical spine injury in patients following blunt trauma: a systematic review. CMAJ. 2012;184:E867–E876. 10.1503/cmaj.120675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Morvan D, Leroy-Willig A. Simultaneous measurements of diffusion and transverse relaxation in exercising skeletal muscle. Magn Reson Imaging. 1995;13:943–948. [DOI] [PubMed] [Google Scholar]
- 82.Mutso AA, Petre B, Huang L, et al. Reorganization of hippocampal functional connectivity with transition to chronic back pain. J Neurophysiol. 2014;111:1065–1076. 10.1152/jn.00611.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Myran R, Hagen K, Svebak S, Nygaard O, Zwart JA. Headache and musculoskeletal complaints among subjects with self reported whiplash injury: the HUNT-2 study. BMC Musculoskelet Disord. 2011;12:129 10.1186/1471-2474-12-129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Myran R, Kvistad KA, Nygaard OP, Andresen H, Folvik M, Zwart JA. Magnetic resonance imaging assessment of the alar ligaments in whiplash injuries: a case-control study. Spine (PhilaPa 1976). 2008;33:2012–2016. 10.1097/BRS.0b013e31817bb0bd [DOI] [PubMed] [Google Scholar]
- 85.Myran R, Zwart JA, Kvistad KA, et al. Clinical characteristics, pain, and disability in relation to alar ligament MRI findings. Spine (PhilaPa 1976). 2011;36:E862–E867. 10.1097/BRS.0b013e3181ff1dde [DOI] [PubMed] [Google Scholar]
- 86.Nakashima H, Yukawa Y, Suda K, Yamagata M, Ueta T, Kato F. Abnormal findings on magnetic resonance images of the cervical spines in 1211 asymptomatic subjects. Spine (Phila Pa 1976). 2015;40:392–398. 10.1097/BRS.0000000000000775 [DOI] [PubMed] [Google Scholar]
- 87.Ogawa S, Lee TM, Kay AR, Tank DW. Brain magnetic resonance imaging with contrast dependent on blood oxygenation. Proc Natl Acad Sci U S A. 1990;87:9868–9872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ogawa S, Tank DW, Menon R, et al. Intrinsic signal changes accompanying sensory stimulation: functional brain mapping with magnetic resonance imaging. Proc Natl Acad Sci U S A. 1992;89:5951–5955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.O’Leary S, Jull G, Van Wyk L, Pedler A, Elliott J. Morphological changes in the cervical muscles of women with chronic whiplash can be modified with exercise—a pilot study. Muscle Nerve. 2015;52:772–779. 10.1002/mus.24612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Pallafacchina G, François S, Regnault B, et al. An adult tissue-specific stem cell in its niche: a gene profiling analysis of in vivo quiescent and activated muscle satellite cells. Stem Cell Res. 2010;4:77–91. 10.1016/j.scr.2009.10.003 [DOI] [PubMed] [Google Scholar]
- 91.Panacek EA, Mower WR, Holmes JF, Hoffman JR. Test performance of the individual NEXUS low-risk clinical screening criteria for cervical spine injury. Ann Emerg Med. 2001;38:22–25. 10.1067/mem.2001.116499 [DOI] [PubMed] [Google Scholar]
- 92.Panjabi MM, Pearson AM, Ito S, Ivancic PC, Wang JL. Cervical spine curvature during simulated whiplash. Clin Biomech (Bristol, Avon). 2004;19:1–9. 10.1016/j.clinbiomech.2003.09.006 [DOI] [PubMed] [Google Scholar]
- 93.Penning L Acceleration injury of the cervical spine by hypertranslation of the head. Part I. Effect of normal translation of the head on cervical spine motion: a radiological study. Eur Spine J. 1992;1:7–12. 10.1007/BF00302135 [DOI] [PubMed] [Google Scholar]
- 94.Pettersson K, Hildingsson C, Toolanen G, Fagerlund M, Björnebrink J. Disc pathology after whiplash injury. A prospective magnetic resonance imaging and clinical investigation. Spine (Phila Pa 1976). 1997;22:283–287; discussion 288. [DOI] [PubMed] [Google Scholar]
- 95.Pettersson K, Hildingsson C, Toolanen G, Fagerlund M, Björnebrink J. MRI and neurology in acute whiplash trauma: no correlation in prospective examination of 39 cases. Acta Orthop Scand. 1994;65:525–528. 10.3109/17453679409000906 [DOI] [PubMed] [Google Scholar]
- 96.Pike JA. Neck Injury Biomechanics. Warrendale, PA: SAE International; 2009. [Google Scholar]
- 97.Platzer P, Hauswirth N, Jaindl M, Chatwani S, Vecsei V, Gaebler C. Delayed or missed diagnosis of cervical spine injuries. J Trauma. 2006;61:150–155. 10.1097/01.ta.0000196673.58429.2a [DOI] [PubMed] [Google Scholar]
- 98.Ploner M, Lee MC, Wiech K, Bingel U, Tracey I. Flexible cerebral connectivity patterns subserve contextual modulations of pain. Cereb Cortex. 2011;21:719–726. 10.1093/cercor/bhq146 [DOI] [PubMed] [Google Scholar]
- 99.Reid DC, Henderson R, Saboe L, Miller JD. Etiology and clinical course of missed spine fractures. J Trauma. 1987;27:980–986. [DOI] [PubMed] [Google Scholar]
- 100.Resnick S, Inaba K, Karamanos E, et al. Clinical relevance of magnetic resonance imaging in cervical spine clearance: a prospective study. JAMA Surg. 2014;149:934–939. 10.1001/jamasurg.2014.867 [DOI] [PubMed] [Google Scholar]
- 101.Ritchie C, Hendrikz J, Jull G, Elliott J, Sterling M. External validation of a clinical prediction rule to predict full recovery and ongoing moderate/severe disability following acute whiplash injury. J Orthop Sports Phys Ther. 2015;45:242–250. 10.2519/jospt.2015.5642 [DOI] [PubMed] [Google Scholar]
- 102.Ritchie C, Hendrikz J, Kenardy J, Sterling M. Derivation of a clinical prediction rule to identify both chronic moderate/severe disability and full recovery following whiplash injury. Pain. 2013;154:2198–2206. 10.1016/j.pain.2013.07.001 [DOI] [PubMed] [Google Scholar]
- 103.Ritter C, Hebart MN, Wolbers T, Bingel U. Representation of spatial information in key areas of the descending pain modulatory system. J Neurosci. 2014;34:4634–4639. 10.1523/JNEUROSCI.4342-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Roberge RJ. Cervical spine radiography after blunt trauma. Is it always needed? Postgrad Med. 1993;93:205–209, 212. [DOI] [PubMed] [Google Scholar]
- 105.Ronnen HR, de Korte PJ, Brink PR, van der Bijl HJ, Tonino AJ, Franke CL. Acute whiplash injury: is there a role for MR imaging?--a prospective study of 100 patients. Radiology. 1996;201:93–96. 10.1148/radiology.201.1.8816527 [DOI] [PubMed] [Google Scholar]
- 106.Sacco A, Doyonnas R, Kraft P, Vitorovic S, Blau HM. Self-renewal and expansion of single transplanted muscle stem cells [letter]. Nature. 2008;456:502–506. 10.1038/nature07384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Salamon N, Ellingson BM, Nagarajan R, Gebara N, Thomas A, Holly LT. Proton magnetic resonance spectroscopy of human cervical spondylosis at 3T. Spinal Cord. 2013;51:558–563. 10.1038/sc.2013.31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Schomacher J, Farina D, Lindstroem R, Falla D. Chronic trauma-induced neck pain impairs the neural control of the deep semispinalis cervicis muscle. Clin Neurophysiol. 2012;123:1403–1408. 10.1016/j.clinph.2011.11.033 [DOI] [PubMed] [Google Scholar]
- 109.Silver NC, Lai M, Symms MR, Barker GJ, McDonald WI, Miller DH. Serial magnetization transfer imaging to characterize the early evolution of new MS lesions. Neurology. 1998;51:758–764. 10.1212/WNL.51.3.758 [DOI] [PubMed] [Google Scholar]
- 110.Sinson G, Bagley LJ, Cecil KM, et al. Magnetization transfer imaging and proton MR spectroscopy in the evaluation of axonalinjury: correlation with clinical outcome after traumatic brain injury. AJNR Am J Neuroradiol. 2001;22:143–151. [PMC free article] [PubMed] [Google Scholar]
- 111.Srinivasan A, Goyal M, Al Azri F, Lum C. State-of-the-art imaging of acute stroke. Radiographics. 2006;26 suppl 1:S75–S95. 10.1148/rg.26si065501 [DOI] [PubMed] [Google Scholar]
- 112.Stemper BD, Yoganandan N, Pintar FA. Effects of abnormal posture on capsular ligament elongations in a computational model subjected to whiplash loading. J Biomech. 2005;38:1313–1323. 10.1016/j.jbiomech.2004.06.013 [DOI] [PubMed] [Google Scholar]
- 113.Sterling M, Hendrikz J, Kenardy J, et al. Assessment and validation of prognostic models for poor functional recovery 12 months after whiplash injury: a multicentre inception cohort study. Pain. 2012;153:1727–1734. 10.1016/j.pain.2012.05.004 [DOI] [PubMed] [Google Scholar]
- 114.Sterling M, Jull G, Kenardy J. Physical and psychological factors maintain long-term predictive capacity post-whiplash injury. Pain. 2006;122:102–108. 10.1016/j.pain.2006.01.014 [DOI] [PubMed] [Google Scholar]
- 115.Sterling M, Jull G, Vicenzino B, Kenardy J, Darnell R. Development of motor system dysfunction following whiplash injury. Pain. 2003;103:65–73. 10.1016/S0304-3959(02)00420-7 [DOI] [PubMed] [Google Scholar]
- 116.Sterling M, Kenardy J. Physical and psychological aspects of whiplash: important considerations for primary care assessment. Man Ther. 2008;13:93–102. 10.1016/j.math.2007.11.003 [DOI] [PubMed] [Google Scholar]
- 117.Stiell IG, Clement CM, Grimshaw J, et al. Implementation of the Canadian C-Spine Rule: prospective 12 centre cluster randomised trial. BMJ. 2009;339:b4146 10.1136/bmj.b4146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Stiell IG, Wells GA, Vandemheen KL, et al. The Canadian C-Spine Rule for radiography in alert and stable trauma patients. JAMA. 2001;286:1841–1848. 10.1001/jama.286.15.1841 [DOI] [PubMed] [Google Scholar]
- 119.Stroman PW, Wheeler-Kingshott C, Bacon M, et al. The current state-of-the-art of spinal cord imaging: methods. Neuroimage. 2014;84:1070–1081. 10.1016/j.neuroimage.2013.04.124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Sullivan MJ, Adams H, Martel MO, Scott W, Wideman T. Catastrophizing and perceived injustice: risk factors for the transition to chronicity after whiplash injury. Spine (Phila Pa 1976). 2011;36:S244–S249. 10.1097/BRS.0b013e3182387fed [DOI] [PubMed] [Google Scholar]
- 121.Tatsumi R, Liu X, Pulido A, et al. Satellite cell activation in stretched skeletal muscle and the role of nitric oxide and hepatocyte growth factor. Am J Physiol Cell Physiol. 2006;290:C1487–C1494. 10.1152/ajpcell.00513.2005 [DOI] [PubMed] [Google Scholar]
- 122.Ulbrich EJ, Aeberhard R, Wetli S, et al. Cervical muscle area measurements in whiplash patients: acute, 3, and 6 months of follow-up. J Magn Reson Imaging. 2012;36:1413–1420. 10.1002/jmri.23769 [DOI] [PubMed] [Google Scholar]
- 123.Walton DM, MacDermid JC, Russell E, Koren G, Van Uum S. Hair-normalized cortisol waking response as a novel biomarker of hypothalamicpituitary-adrenal axis activity following acute trauma: a proof-of-concept study with pilot results. Pain Res Treat. 2013;2013:876871 10.1155/2013/876871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Walton DM, Pretty J, MacDermid JC, Teasell RW. Risk factors for persistent problems following whiplash injury: results of a systematic review and meta-analysis. J Orthop Sports Phys Ther. 2009;39:334–350. 10.2519/jospt.2009.2765 [DOI] [PubMed] [Google Scholar]
- 125.Wang YX, Dumont NA, Rudnicki MA. Muscle stem cells at a glance. J Cell Sci. 2014;127:4543–4548. 10.1242/jcs.151209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Weber KA 2nd, Chen Y, Wang X, Kahnt T, Parrish TB. Lateralization of cervical spinal cord activity during an isometric upper extremity motor task with functional magnetic resonance imaging. Neuroimage. 2016;125:233–243. 10.1016/j.neuroimage.2015.10.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Wheeler-Kingshott CA, Stroman PW, Schwab JM, et al. The current state-of-the-art of spinal cord imaging: applications. Neuroimage. 2014;84:1082–1093. 10.1016/j.neuroimage.2013.07.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Winkelstein BA, McLendon RE, Barbir A, Myers BS. An anatomical investigation of the human cervical facet capsule, quantifying muscle insertion area. J Anat. 2001;198:455–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Winkelstein BA, Nightingale RW, Richardson WJ, Myers BS. The cervical facet capsule and its role in whiplash injury: a biomechanical investigation. Spine (Phila Pa 1976). 2000;25:1238–1246. [DOI] [PubMed] [Google Scholar]
- 130.Winkelstein BA, Rutkowski MD, Sweitzer SM, Pahl JL, DeLeo JA. Nerve injury proximal or distal to the DRG induces similar spinal glial activation and selective cytokine expression but differential behavioral responses to pharmacologic treatment. J Comp Neurol. 2001;439:127–139. 10.1002/cne.2000 [DOI] [PubMed] [Google Scholar]
- 131.Worsley KJ . Statistical analysis of activation images In: Jezzard P, Matthews PM, Smith SM, eds. Functional MRI: An Introduction to Methods. Oxford, UK: Oxford University Press; 2001:ch 14. [Google Scholar]
- 132.Yamada M, Tatsumi R, Kikuiri T, et al. Matrix metalloproteinases are involved in mechanical stretch-induced activation of skeletal muscle satellite cells. Muscle Nerve. 2006;34:313–319. 10.1002/mus.20601 [DOI] [PubMed] [Google Scholar]
- 133.Yanagisawa O, Kurihara T. Intramuscular water movement during and after isometric muscle contraction: evaluation at different exercise intensities. Clin Physiol Funct Imaging. 2016;36:368–375. 10.1111/cpf.12239 [DOI] [PubMed] [Google Scholar]
- 134.Yanagisawa O, Kurihara T, Fukubayashi T. Alterations in intramuscular water movement associated with mechanical changes in human skeletal muscle fibers: an evaluation using magnetic resonance diffusion-weighted imaging and B-mode ultrasonography. Acta Radiol. 2011;52:1003–1008. 10.1258/ar.2011.110153 [DOI] [PubMed] [Google Scholar]
- 135.Yanagisawa O, Shimao D, Maruyama K, Nielsen M. Evaluation of exercised or cooled skeletal muscle on the basis of diffusion-weighted magnetic resonance imaging. Eur J Appl Physiol. 2009;105:723–729. 10.1007/s00421-008-0954-9 [DOI] [PubMed] [Google Scholar]
- 136.Yin H, Price F, Rudnicki MA. Satellite cells and the muscle stem cell niche. Physiol Rev. 2013;93:23–67. 10.1152/physrev.00043.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]


