Contrary to global production, most fibers floating in the ocean are not synthetic but natural fibers of animal or plant origin.
Abstract
Microfibers are ubiquitous contaminants of emerging concern. Traditionally ascribed to the “microplastics” family, their widespread occurrence in the natural environment is commonly reported in plastic pollution studies, based on the assumption that fibers largely derive from wear and tear of synthetic textiles. By compiling a global dataset from 916 seawater samples collected in six ocean basins, we show that although synthetic polymers currently account for two-thirds of global fiber production, oceanic fibers are mainly composed of natural polymers. µFT-IR characterization of ~2000 fibers revealed that only 8.2% of oceanic fibers are synthetic, with most being cellulosic (79.5%) or of animal origin (12.3%). The widespread occurrence of natural fibers throughout marine environments emphasizes the necessity of chemically identifying microfibers before classifying them as microplastics. Our results highlight a considerable mismatch between the global production of synthetic fibers and the current composition of marine fibers, a finding that clearly deserves further attention.
INTRODUCTION
Global fiber production, both synthetic and natural, has more than doubled in the past 20 years, reaching 107 million metric tons (MMT) in 2018 and is expected to reach 145 MT in 2030 if business as usual continues (1). Largely driven by the production of polyester (55 MT year−1 in 2018), synthetic polymers have dominated the textile market since the mid-1990s when they overtook cotton as the dominant fiber type. Synthetic fibers now account for almost two-thirds of global fiber production (2) and for 14.5% of plastic production by mass (3). With a market share of 24% and 15% growth from 2017 to 2018 (1), cotton is the second most important fiber (27 MT year−1 in 2018), followed by man-made cellulosics (e.g., viscose/rayon), which account for 6.2% of global fiber production (6.7 MT year−1). Other plant-based fibers, such as jute, linen, and hemp, together account for 5.7% of the global market (6 MT year−1), while animal fibers (wool and silk) account for just over 1% of annual production (1). The main uses of both natural and synthetic fibers are clothing and apparel, followed by household and furnishings, automotive, and other industrial applications such as construction, filtration, and personal care (4). Shedding of fabric materials, wear and tear, and increased consumption have led to the accumulation of these fibers in the natural environment (5, 6).
Large numbers of fibers are discharged into wastewater from washing clothes (7–9), with each garment releasing up to 107 fibers per wash (10–12), and enter the environment through wastewater effluent (13), aerial deposition (14, 15), or through the application of contaminated sludge on agricultural soils (16). As a consequence, fibers are now the most prevalent type of anthropogenic particle found by microplastic pollution surveys around the world (17), including human exposure studies (18). Substantial concentrations have been detected in surface and subsurface waters (19–21), in sea ice (22), deep-sea (23, 24) and coastal sediments (25), as well as in terrestrial and freshwater ecosystems (26, 27). Given their abundance, it is not surprising that fibers have been detected in food (18), drinking water (28), and human lungs (29), as well as in the digestive tracts of many aquatic (30, 31) and terrestrial organisms (32). Under laboratory conditions, adverse health effects due to ingestion of microfibers have been observed in marine (33), freshwater (34), and terrestrial (35) invertebrates, but no proof of harm is currently available for wild organisms exposed to environmentally relevant fiber concentrations. In addition, a wide variety of chemicals are used during textile production including dyes, additives, and flame retardants (4), raising concerns about the role of fibers as vectors of hazardous substances into the environment (7).
Because of the risk of external contamination (36), fibers were initially excluded from microplastic surveys (37). However, they are now commonly included in many studies, often accounting for 80–90% of microplastic counts (7, 17), although their synthetic nature is seldom demonstrated. Here, we show that synthetic polymers only account for a small portion of the fibers extracted from open ocean samples. Besides emphasizing the need for further research on the fate and impacts of natural fibers in marine ecosystems, our results indicate that, in the absence of a comprehensive chemical characterization, the abundance of microplastic fibers in natural environments has been probably overestimated. Our results highlight a considerable mismatch between the global production of synthetic textiles and the current composition of marine fibers, a finding that deserves further research.
RESULTS
Our dataset consists of 916 seawater samples collected at 617 locations in six oceanic basins (13,447.5 liters of seawater filtered). Fibers were found in 99.7% of all samples, totaling 23,593 fibers (median 18 fibers per sample; Q1–Q3, 10–31 fibers). Fibers were absent from only three samples: one subsurface sample collected in the North Atlantic and two samples collected off the coast of Mozambique in the Indian Ocean. The raw concentration of fibers spanned three orders of magnitude from 0.02 to 25.8 fibers liter−1 (Fig. 1) with a median concentration of 1.7 fibers liter−1, which decreased to 1.6 fibers liter−1 (Table 1) after applying correction factors for contamination level, sampling depth, and mesh size effect (table S1 and fig. S1). Most fibers were dark/black (57.1%) or light/gray (24.2%), followed by blue (10.1%), red/orange (5.2%), yellow/amber (2.9%), and green (0.4%).
Fig. 1. The raw concentration of fibers collected at the ocean surface.
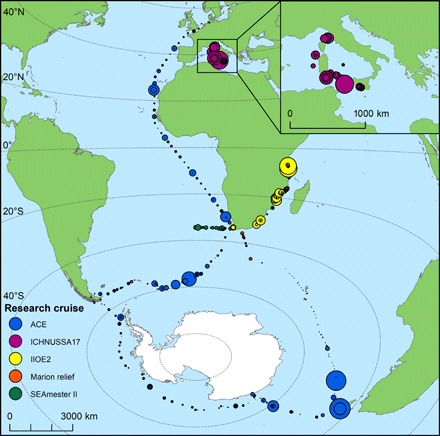
Circle size is scaled by fiber density, which ranges from 0 to 25.8 fibers liter−1. Data sources are provided in the Supplementary Materials (n = 916 seawater samples). See table S5 for more details about sampling campaigns. ACE, Antarctic Circumnavigation Expedition; IIOE2, Second International Indian Ocean Expedition.
Table 1. Fiber concentrations.
Median value and interquartile range (Q1–Q3) of fiber concentrations (fibers liter−1) found in all oceanic basins and sub-basins sampled during this survey. Both raw and corrected data are shown (n, number of samples collected in each basin).
| Uncorrected | Corrected | ||||
| n | Median | Q1–Q3 | Median | Q1–Q3 | |
| Mediterranean Sea | 108 | 4.2 | 3.0–6.4 | 4.6 | 3.0–7.7 |
| Indian Ocean | 304 | 1.8 | 0.9–3.2 | 1.2 | 0.3–3.0 |
| Atlantic Ocean | 241 | 1.2 | 0.4–2.1 | 1.0 | 0.1–2.2 |
| North | 91 | 0.8 | 0.2–1.7 | 0.6 | 0.0–1.9 |
| South | 150 | 1.5 | 0.7–2.3 | 1.3 | 0.2–2.5 |
| Southern Ocean | 263 | 1.2 | 0.5–2.5 | 1.7 | 0.4–3.5 |
| 40–60°S | 169 | 1.1 | 0.5–2.7 | 1.6 | 0.3–3.9 |
| >60°S | 94 | 1.3 | 0.6–2.2 | 1.7 | 0.5–2.9 |
| All | 916 | 1.7 | 0.7–3.1 | 1.6 | 0.4–3.5 |
Fiber concentration was not homogeneous across ocean basins [Kruskall-Wallis H(χ2) = 140.1, P = 2.5 × 10−41). The highest concentrations were found in the Mediterranean Sea (Fig. 2), while the North Atlantic Ocean exhibited significantly lower concentrations than all other basins (Table 1). The concentration of fibers tended to increase from north to south, but the latitudinal range sampled was greater in the southern hemisphere, and this pattern may reflect a general increase in fiber concentrations at higher latitudes (fig. S2). When excluding Mediterranean samples, a significant negative correlation was found between latitude and corrected fiber concentration [rs(806) = –0.16, P = 2.3 × 10−6]. This correlation was also significant when the dataset was restricted to a single cruise [Antarctic Circumnavigation Expedition (ACE)], both including the North Atlantic [rs(406) = –0.23, P = 3.6 × 10−6] and only samples collected in the southern hemisphere [rs(715) = –0.11, P = 0.0021]. High fiber concentrations were found in the Southern Ocean between 40°S and 60°S (Fig. 2 and Table 1), suggesting accumulation of fibers north of the polar front or retention within the Antarctic Circumpolar Current. Nevertheless, the concentration of fibers south of 60°S was not significantly different from that found in the South Atlantic or the Indian Ocean (table S2).
Fig. 2. The concentration of microfibers found in different ocean basins.
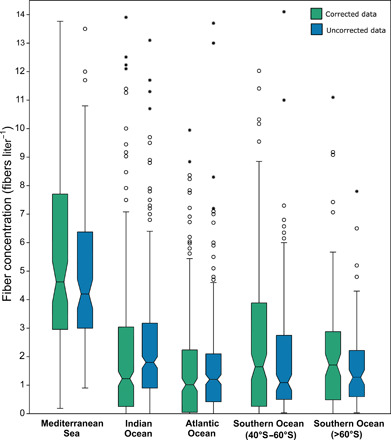
Corrected (green) and uncorrected (blue) fiber concentrations found in the main five basins and sub-basins surveyed during this study. Boxes show 25–75 percentiles with median values as central lines. Whiskers denote upper [Q3 + (1.5 × interquartile range)] and lower [Q1 − (1.5 × interquartile range)] inner fences. Values outside them are shown as circles, while stars denote values exceeding outer fences (i.e., 3 × interquartile range). Values >15 fibers liter−1 (n = 30) are not shown for clarity.
Median fiber length was 1.07 mm (Q1–Q3, 0.65–1.74 mm; range, 0.09–27.06 mm). Only 10 fibers were longer than 10 mm, and only 3 fibers were longer than 15 mm. Fiber length distribution showed a peak in abundance around 0.8–0.9 mm and a pronounced gap below 0.4 mm (Fig. 3A). Significant differences were found in fiber length among ocean basins [Kruskal-Wallis H(χ2) = 43.9, P = 1.6 × 10−9), with fibers from the Mediterranean Sea being significantly longer and fibers from the Southern Ocean being significantly shorter than all other basins (Table 2). Fibers from the Indian and the Atlantic Ocean were of intermediate length and not significantly different from each other (table S3A).
Fig. 3. Length and diameter of the fibers.
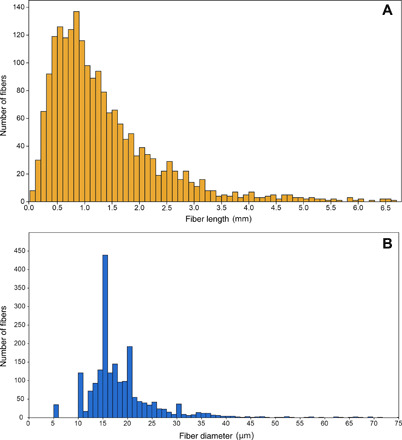
The frequency distribution of fiber lengths (A) and diameters (B) measured across the entire dataset (n = 2016 fibers) and pooled in 0.1 mm (A) and 1 μm (B) bins, respectively. Fibers longer than 7 mm (n = 24) and thicker than 75 μm (n = 10) are not shown for clarity.
Table 2. Fiber dimensions.
Median value and interquartile range (Q1–Q3) of fiber length (millimeters) and diameter (micrometers) measured in a subset of fibers (n) extracted from seawater and blank samples.
| Fiber length (mm) | Fiber diameter (μm) | ||||
| n | Median | Q1–Q3 | Median | Q1–Q3 | |
| Mediterranean Sea | 336 | 1.33 | 0.76–2.03 | 18.6 | 15.0–22.9 |
| Indian Ocean | 342 | 1.06 | 0.65–1.75 | 16.8 | 15.0–20.6 |
| Atlantic Ocean | 338 | 1.11 | 0.71–1.85 | 15.0 | 14.9–20.0 |
| Southern Ocean | 1000 | 0.96 | 0.60–1.55 | 16.7 | 15.0–20.0 |
| All | 2016 | 1.07 | 0.65–1.74 | 16.7 | 15.0–20.4 |
| Blanks | 161 | 1.01 | 0.61–2.01 | 15.8 | 14.2–19.9 |
Median fiber diameter was 16.7 μm (Q1–Q3, 15.0–20.4 μm; range, 5 to 239 μm). Most fibers had a diameter of 10–11 μm, 15–17 μm, or 19–20 μm (Fig. 3B). Median aspect ratio (length:diameter) was 62.9 (Q1–Q3, 35.7–104.8; range, 3.1 to 1315). As with fiber length, there were significant differences among ocean basins in fiber diameters [Kruskal-Wallis H(χ2) = 33.09, P = 2.9 × 10−7], with all basins being significantly different from each other except the Indian and the Southern oceans (table S3). Fibers from the Mediterranean Sea were significantly thicker than those collected in the Southern, Indian, and Atlantic oceans (Table 2 and fig. S3B).
When compared to seawater samples, the fibers extracted from procedural blanks (n = 161) were significantly thinner (Mann-Whitney U = 1.45 × 10−5, P = 0.0238) but not shorter (Mann-Whitney U = 1.61 × 10−5, P = 0.911). The same was true when considering all basins separately (Table 2), with the exception of the Mediterranean Sea, where fibers were significantly longer and thicker, and the Indian Ocean, where fibers were significantly thicker than those found in the blanks (fig. S3 and table S3).
Micro–Fourier transform infrared (μFTIR) characterization revealed that 91.8% of fibers were natural fibers of animal or plant origin (n = 1984). Most fibers were cellulosics (79.5%), with cotton being the most frequent match (50% of all fibers; n = 992), followed by other plant-based fibers (e.g., viscose, linen, jute, kenaf, hemp, etc.), which accounted together for 29.5% of all fibers (n = 585). A further 12.3% (n = 244) were animal fibers: 11.6% wool and 0.6% silk. Only 8.2% of fibers were synthetic (n = 163). Most plastic fibers were polyester (n = 123; 6.2% of the total), followed by acrylic and nylon (n = 14 each; 0.7%), polypropylene (n = 7; 0.4%), and aramid (n = 5; 0.3%).
The composition of fibers was not homogeneous across ocean basins (χ2 = 46.89, df = 12, P = 9.04 × 10−9). The relative proportion of synthetic fibers increased at higher latitudes, from 6.8% in the Mediterranean to a maximum of 12.6% in Antarctic waters south of 60°S (Fig. 4). A similar pattern was found for animal fibers, whose contribution in the Southern Ocean (14.5%) was almost double that in the Indian Ocean (7.7%) and the Mediterranean Sea (6.3%). There was a corresponding decrease in the occurrence of cellulosic fibers at higher latitudes from a maximum of 86.9% in the Mediterranean to 72.9% in Antarctic waters. The composition of fibers extracted from blank samples (n = 150) was significantly different from seawater samples (χ2 = 7.987, df = 2, P = 0.0092), because of a higher proportion of cellulosic fibers (87.3%) and a shortage of wool fibers (4.7%), but similar content of synthetic fibers (8%). All the synthetic fibers from the Indian Ocean (n = 24) were made of polyester, while polypropylene and aramid were found only in the Mediterranean and in the Southern Ocean, although in low numbers (table S4).
Fig. 4. The composition of the fibers extracted from blank and seawater samples.
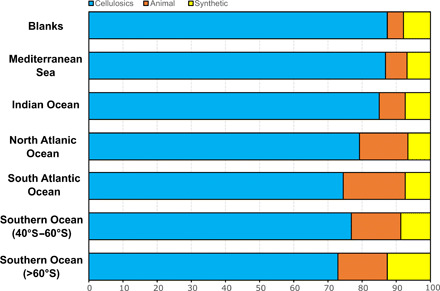
Results of the μFTIR analysis showing the composition of all fibers collected in six oceanic basins compared to laboratory blanks (n = 2134 fibers). More details are given in table S4.
DISCUSSION
Most fibers floating in the world’s oceans are not plastic but dyed cellulose. This is in agreement with recent studies showing that cellulosic fibers account for more than 60–80% of all fibers in seafloor sediments (23, 24), marine organisms (30, 31), wastewater (38), fresh water (26, 27), ice cores (22), and airborne fiber populations (14, 39). Before these studies, cellulosic fibers (natural and regenerated) have been likely included in the synthetic realm by hundreds of studies, inflating “microplastic” counts in both environmental matrices and organisms (7, 40). This error has resulted either from the assumption that all colored fibers are synthetic—although without proper chemical identification, visual identification of synthetic fibers has been criticized (30)—or from the assumption that man-made cellulosic fibers can be considered synthetic and included in microplastic counts because they are extruded and processed industrially (22, 23). A previous large-scale investigation, for instance, reported that 69% of marine fibers were synthetic (21). However, this study was based on the characterization of barely 100 fibers and classified man-made cellulosics in the synthetic category, although it is extremely challenging to distinguish between natural and regenerated cellulose by FTIR techniques (41, 42). Visual and chemometric methods are being developed (43, 44), but the presence of dyes (30), oxidation, and microbial degradation can alter cellulose absorption bands (11, 45), making it very difficult to distinguish natural and man-made cellulose, especially when dealing with environmentally degraded polymers (46). “Semi-synthetic” cellulosics are a readily available source of carbon for microorganisms (11), and their biodegradability seems to be similar to (11), if not faster than, cotton yarns (47), and considerably faster than synthetic polymers such as polyester (45). Therefore, as already highlighted by other authors (7, 46), extruded cellulosics such as viscose/rayon, lyocell, and acetate should not be considered synthetic, irrespective of their origin (42).
The high proportion of animal- and plant-based fibers throughout the world’s oceans is unexpected, given the dominance of synthetic fibers in current global production (62%, compared to 8% in our samples). Cellulosic and animal fibers accounted for 80 and 12% of our samples, despite comprising only 36% and less than 2% of global production, respectively (1). This contrasts with the pattern of macro- and microplastic litter, where polyethylene and polypropylene are the two most common polymers both at sea (48) and produced by the plastic industry (3). The specific density of cotton/cellulose (1.54–1.63 g cm−3) is higher than that of polyester (1.37–1.46 g cm−3), polypropylene (0.9 g cm−3), nylon/acrylics (1.14–1.18 g cm−3), and aramid fibers (1.38–1.47 g cm−3), so if anything, cellulosic fibers should be underrepresented rather than overrepresented in surface water samples. The causes of this apparent shortage of synthetic fibers in environmental samples are currently unknown, and more research is needed to elucidate this pattern. One plausible explanation is that wool, cotton, and rayon fabrics shed and release more fibers than polyester during laundering (8, 9, 11, 12). However, a crucial factor to understand is the life span of different fiber types in the environment, given the historical dominance of plant and animal fiber use in textiles (2). Despite being considered biodegradable (49), little is known about the degradation of wool and cellulosic fibers in marine environments (50). Rayon and cotton yarns are often processed, finished, dyed, and coated with a wide range of chemicals including resins, softeners, and flame retardants, which may considerably slow their remineralization (45), to the extent that a dyed cotton waistcoat recovered from a deep-ocean shipwreck showed almost no sign of degradation after 133 years of submersion (51). Together, these factors may explain the long-term accumulation of cellulosic fibers in marine environments.
The high variability in fiber densities among replicate samples collected at the same location (52) stresses the need for caution when estimating global fiber abundances or assessing regional differences in fiber densities. This uncertainty is exacerbated by the use of different mesh sizes and sample depths across our large-scale study. We attempted to compensate for the effects of mesh size and sample depth (see the Supplementary Materials), but the innate variability among samples means that our density estimates should be regarded as indicative of comparative densities rather than precise estimates. Bearing these caveats in mind, a notable result is the relatively limited regional variation in fiber densities among ocean basins, with little correlation with proximity to likely source areas. Our results suggest that the mean concentration of fibers in the surface oceans is in the order of ~108 fibers km−2 (assuming that 1 liter is equivalent to 10 × 10 × 10 cm). Extrapolating this figure across all the world’s oceans translates to approximately 9 × 104 to 38 × 104 metric tons of fibers in the top meter of the world’s oceans (see Materials and Methods below). This is the same order of magnitude as recent estimates of floating plastics (i.e., 9 × 104 to 24 × 104 metric tons) (53). However, because synthetic fibers are less abundant than cellulosics, they would only add an additional ∼6 to 12% to the total load of plastic floating in the surface 1 m of the global ocean (i.e. 6 × 103 to 29 × 103 metric tons). In this regard, between 18 thousand (54) and 520 thousand (55) metric tons of fibers are estimated to enter the ocean every year. This equates to an average global input of 1.3 × 106 metric tons in the past 5 years. Thus, according to our estimates, substantial amounts of fibers are probably exported to the seafloor (23, 24), suspended in the water column (19, 20), or ingested by biota (31). With the exception of polypropylene, all natural and synthetic polymers found in our study have densities greater than seawater and should sink (56). Their widespread occurrence in surface waters could thus be explained by a constant atmospheric deposition to the ocean surface (15), coupled with retention mechanisms within the sea surface microlayer and complex turbulence and resuspension processes (19) about which we know very little.
Being a densely populated, enclosed basin, it is not surprising that the highest fiber concentrations were found in the Mediterranean Sea, which has some of the highest microplastic densities in the world (48). Unexpectedly, high concentrations were also found in the Southern Ocean (21), often regarded as a pristine environment. In addition to local inputs from visiting vessels and research stations (57), high abundances of fibers in this remote region are probably linked to long-range marine and aerial transport (15), as demonstrated for other anthropogenic contaminants (58), coupled with slow degradation of organic materials in cold-water environments (51). The diameters of our fibers closely matched those of commonly manufactured textile fibers (10, 12). Nevertheless, the longest fibers were found in the Mediterranean Sea, probably indicating recent inputs into the basin, being closer to pollution sources and more similar to the length of cotton and rayon fibers freshly released by laundering (10, 11). At sea, fibers will be progressively broken down, which might explain the shorter and thinner fibers found in the Southern Ocean, relatively far from main sources. Alternatively, smaller fibers may be most easily advected by long-range aerial transport, thus reaching the most remote areas of the planet (15). Thinner fibers were found in blank samples, and short fibers (usually between 100 and 700 μm) are found in atmospheric fallout (14, 15, 44), notably shorter than those found in our study or in laundry experiments (10, 11). Future studies focusing on providing reliable weathering and aging indicators for cellulosic and synthetic fibers will be highly beneficial to better understand fiber degradation and residence time in the marine environment.
The use of natural fibers is being advocated as a strategy to reduce inputs and risks of microplastics into the environment (50). However, animal and cellulosic fibers are greatly underrepresented in environmental pollution literature (39). Research on the prevalence, fate, and impacts of microfibers is relatively young and often unbalanced in favor of plastic polymers. More information is needed on the degradation of natural fibers relative to synthetic polymers. Here, we show that natural and synthetic fibers are ubiquitous in the world’s oceans and that their abundance and composition are not homogeneous among ocean basins. As already demonstrated in freshwater and atmospheric deposition (39) and in marked contrast to global production patterns, around 80–90% of fibers in our samples are of natural origin. Understanding the ecological impacts and biodegradation rates of natural and synthetic fibers in a range of environmental conditions is crucial for assessing their potential impacts on environments and ecosystems worldwide.
MATERIALS AND METHODS
Seawater sampling
We collected 916 seawater samples during five research cruises carried out between January and November 2017 (Fig. 1 and table S5): ACE (Antarctic Circumnavigation Expedition, from January to April 2017, with legs 1 to 3 in the Southern Ocean and leg 4 in the Atlantic Ocean from South Africa to Germany), Marion Island relief voyage (Cape Town–Marion Island, May 2017), SEAmester II (Southeast Atlantic Ocean, June 2017), Second International Indian Ocean Expedition (IIOE2) (Western Indian Ocean between South Africa and Tanzania, October to November 2017), and ICHNUSSA17 (Western Mediterranean Sea, October to November 2017). At each sampling station, replicate samples (n = 2–4) were collected to overcome low sampling repeatability (52). Samples were primarily collected using a bucket to sample the sea surface (n = 710), although paired subsurface samples were collected from the ship’s underway water supply during ACE and the Marion Island relief voyage (n = 206). The intake for these samples was approximately 5 m below the hull on both the research vessel Akademik Tryoshnikov (ACE) and SA Agulhas II (Marion Island relief voyage).
Bulk-water samples were collected using a 15-liter stainless steel bucket, triple rinsed in seawater before use, and immediately poured into prewashed 10-liter containers for transfer to an on-board filtration laboratory. To minimize external contamination, all sampling equipment was prewashed with filtered water before use, and containers were kept covered at all times during transfer to the laboratory (see next section for more details on contamination control). To exclude disturbance and contamination from the ship itself (e.g., from wastewater outlets), bucket samples were typically collected from the ship’s forecastle deck while the ship was slowly moving forward (e.g., at 2 to 3 knots during neuston net tows). On some vessels, it was possible to collect samples from the bow at speeds of up to 15 knots. However, on the SA Agulhas II, it was not safe to sample from the bow during bad weather, and so a few samples were collected alongside the ship, ahead of the bow wave.
All replicate samples were gravity-filtered soon after collection through 20-μm (n = 148), 25-μm (n = 299), 37-μm (n = 265), 50-μm (n = 38), or 63-μm (n = 73) nylon mesh filters or vacuum-filtered through 0.7-μm microglass fiber filters (Ø 47 mm; Munktell; n = 93). Sample size was generally 10 liters. However, in 45 samples (4.9% of the total) collected in the Southern, Indian, and Atlantic oceans, the finer filters (<37 μm) became clogged before a full sample could be processed; when this occurred, the filtered volume was noted (minimum, 3 liters), and the density of fibers was computed accordingly. Because samples were filtered immediately after collection, there was little risk of fibers aggregating within samples and thus being affected by incomplete sample processing. However, we obtained broadly the same patterns if these incomplete samples were removed from our dataset. Subsurface samples (n = 206) were filtered directly from the ship’s underway pump, sampling 10 liters or until the filter clogged (median, 30 liters; maximum, 105 liters). Upon collection, all filters were placed in clean foil envelopes or Ø 47-mm petri dishes, labeled, and stored frozen at −20°C. In the laboratory, samples were examined by the same individual under a stereomicroscope (Leica MZ16) at ×45 magnification. All fibers were counted and classified according to standard criteria, i.e., uniform thickness, lack of cellular structure, coloration, and high tensile strength (21, 55). Raw fiber concentrations were then computed for all samples and expressed as fibers per liter.
Contamination control
The levels of airborne contamination were measured by placing moistened filters next to the microscope during sorting and sample handling in the laboratory as well as during sampling operations onboard. Wet filters were left exposed in petri dishes to the open air during the entire sampling and processing procedure and then closed, labeled, and stored in the freezer. All control samples were examined following the same procedure used for seawater samples. Mesh filters, laboratory ware, and sampling equipment were triple rinsed with filtered water before use, and samples were kept covered as much as possible during sampling and processing (36, 37). Procedural blanks were performed by filtering 10 liters of Milli-Q ultrapure water (Merck) through the same equipment used for sampling. Aerial control samples accumulated 2.0 ± 3.2 fibers hour−1 (median, 1.0 fiber hour−1; n = 125), suggesting low airborne contamination during sampling (i.e., ∼0.2 to 0.3 fibers per sample, given that processing took 5 to 10 min per sample and that samples were always kept covered during filtering and sorting procedures). Procedural blanks indicated a greater contamination risk (1.1 ± 1.1 fibers liter−1; median, 0.65 fibers liter−1; n = 22) but still significantly lower than environmental concentrations (Mann-Whitney, P = 0.00033). Consequently, to compensate for external contamination, all samples were conservatively reduced by 1.0 fibers liter−1, and all negative values were set to 0.
Correction factors for mesh size, sampling depth, and volume
When comparing multiple paired samples from the same locations filtered through different mesh sizes (table S5), fine filters tended to capture more fibers than coarse filters [see Supplemental Text and (52) for more details]. To compensate for this effect, crude retention coefficients of different mesh sizes were estimated by linear interpolation assigning 100% retention score to the 0.7-μm mesh (table S1). Data from (52) were then fitted (initially by log-transforming y, followed by nonlinear optimization) to an exponential function (y = aebx + c), selected according to the lowest Akaike information criterion (AIC) (fig. S1). Correction factors (table S1) were estimated from this model and applied to all samples to compensate for the effect of mesh size on fiber retention.
Sampling depth and volume (for coarser mesh filters) also had a significant effect on fiber concentration (52). Surface samples contained, on average, 2.4 times more fibers than paired subsurface samples filtered on the same mesh (n = 21), so a crude correction factor of 2.4 was applied to all subsurface samples to compensate for this effect (see Supplementary Text for more details). Given the crude nature of these correction factors, both raw and corrected values are presented throughout the text and provided in the data supplement.
μFTIR analysis
A total of 2134 fibers were analyzed by μFTIR techniques: 1984 from field samples and 150 from procedural blanks. Fibers were randomly selected from a subset of 224 samples equally distributed among different cruises and ocean basins (table S4). Subsamples were selected to provide a roughly equal coverage from each of the major water masses sampled (North Atlantic, South Atlantic, Indian Ocean, Southern Ocean, and Mediterranean Sea). Similarly, within each filter, fibers were selected systematically from a random starting point to avoid any subconscious bias in fiber selection. The proportion of fibers analyzed per sample varied from 2 to 100% (mean, 48%), but typically, a fixed number of fibers were extracted from each filter, unless the sample lacked sufficient fibers to do so (median, 10 fibers per sample; range, 1 to 14 fibers). Although we did our best to truly randomize the selection of the fibers to be analyzed, a bias toward cellulosic fibers could have been introduced during manual extraction of fibers from our samples. Synthetic fibers, however, are generally smoother and brighter than cellulosic ones (55), and therefore, the bias should have been directed toward a preferential selection of synthetic fibers, rather than cellulosic ones.
Individual fibers were handpicked from the filters using ultrafine laboratory tweezers and placed on moistened glass slides to promote adhesion on the horizontal plane. Slides were kept covered to prevent airborne contamination and left to dry in a laboratory oven for 5 hours at 35°C. All analyses were performed at CNR-ISMAR laboratories using a LUMOS stand-alone FTIR microscope (Bruker Optik GmbH), equipped with a motorized XY sample stage, and operated in attenuated total reflection (ATR) mode (Ge crystal). Before each scan, fiber length and diameter were measured to the nearest 1 μm from digital images. Following background scans, ATR spectra were obtained by averaging 64 scans per item with a spectral resolution of 4 cm−1 (range, 4000–650 cm−1). After acquisition, infrared spectra were processed and analyzed using OPUS 7.5 software. CO2 interference (adsorption at 2300–2400 cm−1) was removed for clarity, and polymer identification was performed by comparison with a combination of commercially available libraries and an additional custom library compiled within the framework of the JPI Oceans project BASEMAN (38). To further increase identification accuracy, FTIR spectra of common natural and synthetic fabrics, clothing, and textiles were obtained and added to the spectral database according to their label information. Sample spectra were compared to the augmented database (first-derivative, vector-normalized), and only matches >75–80% with reference spectra were accepted as verified polymers. Fibers were classified as synthetic (polyester, acrylic, polyamides, aramids, and polypropylene), animal (wool and silk) or cellulosic fibers, consisting of both natural (cotton, linen, and other plant-based fibers such as jute, kenaf, hemp, flax, and sisal) and man-made cellulosics (e.g., rayon/viscose, modal, acetate, and lyocell). Although some methods are being developed to distinguish between natural and man-made cellulosic fibers (43, 44), this is extremely challenging because these two polymers have almost identical FTIR spectra (41), so we made no attempt to distinguish between them (46).
Data analysis
The dataset was divided into six oceanic basins and sub-basins: the Mediterranean Sea (n = 108 samples), the western Indian Ocean (n = 304, mainly from the Mozambique channel and off the east coasts of South Africa and Tanzania), the North (n = 91) and South (n = 150) Atlantic oceans and the Southern Ocean (n = 169 in oceanic waters between 40°S and 60°S and n = 94 in Antarctic waters >60°S). Data distribution was non-normal (Shapiro-Wilk W = 0.4841, P = 2.61 × 10−45); therefore, nonparametric tests (Mann-Whitney, Kruskal-Wallis, chi-squared goodness-of-fit) were used to test differences in fiber concentrations, length, diameter, and composition across oceanic basins and sub-basins. Correlations were tested using Spearman’s rank-order correlation, and the level of statistical significance was set at P < 0.05.
Global estimate of floating fiber load
A first-order global estimate of the amount of fibers floating in the world’s ocean was obtained using the 25–75% confidence interval of our uncorrected dataset (Table 1) extrapolated to the top 1 m of the world’s ocean (3.6 × 1017 liters). The weight of fibers was computed using the specific density of all polymers (cellulose, 1.5 g cm−3; wool/silk, 1.3 g cm−3; polyester, 1.4 g cm−3; nylon/acrylic, 1.15 g cm−3; polypropylene, 0.9 g cm−3; and aramid, 1.47 g cm−3), assuming a cylindrical shape approximated to their respective median fiber length and diameter (Table 2). These values were then multiplied by the total estimated number of fibers in each polymer class, computed according to their relative abundance in our dataset (table S4).
Supplementary Material
Acknowledgments
We thank D. Bassotto, D. Bergstrom, M. Borghini, L. Beloša, R. Daling, R. Flynn, H. Forrer, K. Kiefer, J. Mang’ena, M. Musso, A. Osborne, S. Schoombie, M. M. Taque, M. van den Berg, S. Waterworth, and the crews of research vessels Akademik Tryoshnikov, SA Agulhas II, and Minerva Uno for assistance during field and laboratory work. Funding: ACE was a research cruise of the Swiss Polar Institute, supported by funding from the ACE Foundation. The South African Departments of Environmental Affairs and Science and Technology and the South African National Antarctic Programme provided logistic support on the IIOE2, SEAmester II, and 2017 Marion Island relief cruises. Additional funding was provided by the JPI Oceans project BASEMAN (defining the baselines and standards for microplastics analyses in European waters). Author contributions: G.S., P.G.R., S.A., and T.G.B. participated in the design and coordination of field surveys. G.S., P.G.R., T.G.B., A.P., and V.P. collected the samples. G.S. and A.A. performed laboratory work. G.S., J.R.L., and P.G.R. analyzed the data. G.S. and P.G.R. wrote the manuscript, and all authors reviewed and approved it. Competing interests: The authors declare that they have no competing interests. Data and materials availability: All data needed to evaluate the conclusions of this study are freely available without restrictions as a data supplement to this article. Additional data related to this paper may be requested from the authors.
SUPPLEMENTARY MATERIALS
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/6/23/eaay8493/DC1
REFERENCES AND NOTES
- 1.Textile Exchange, “Preferred fiber & materials—Market report 2019,” Tech. rep. (2019).
- 2.The Fiber Year, “World survey on textiles & nonwovens. Issue 18,” Tech. rep. (2018).
- 3.Geyer R., Jambeck J. R., Law K. L., Production, use, and fate of all plastics ever made. Sci. Adv. 3, e1700782 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.R. R. Mather, R. H. Wardman, The Chemistry of Textile Fibres (The Royal Society of Chemistry, 2015). [Google Scholar]
- 5.Carr S. A., Sources and dispersive modes of micro-fibers in the environment. Integr. Environ. Asses. Manag. 13, 466–469 (2017). [DOI] [PubMed] [Google Scholar]
- 6.Liu J., Yang Y., Ding J., Zhu B., Gao W., Microfibers: A preliminary discussion on their definition and sources. Environ. Sci. Pollut. Res. Int. 26, 29497–29501 (2019). [DOI] [PubMed] [Google Scholar]
- 7.Cesa F. S., Turra A., Baruque-Ramos J., Synthetic fibers as microplastics in the marine environment: A review from textile perspective with a focus on domestic washings. Sci. Total Environ. 598, 1116–1129 (2017). [DOI] [PubMed] [Google Scholar]
- 8.Cesa F. S., Turra A., Checon H. H., Leonardi B., Baruque-Ramos J., Laundering and textile parameters influence fibers release in household washings. Environ. Pollut. 257, 113553 (2020). [DOI] [PubMed] [Google Scholar]
- 9.Sillanpää M., Sainio P., Release of polyester and cotton fibers from textiles in machine washings. Environ. Sci. Pollut. Res. Int. 24, 19313–19321 (2017). [DOI] [PubMed] [Google Scholar]
- 10.Napper I. E., Thompson R. C., Release of synthetic microplastic plastic fibres from domestic washing machines: Effects of fabric type and washing conditions. Mar. Pollut. Bull. 112, 39–45 (2016). [DOI] [PubMed] [Google Scholar]
- 11.Zambrano M. C., Pawlak J. J., Daystar J., Ankeny M., Cheng J. J., Venditti R. A., Microfibers generated from the laundering of cotton, rayon and polyester based fabrics and their aquatic biodegradation. Mar. Pollut. Bull. 142, 394–407 (2019). [DOI] [PubMed] [Google Scholar]
- 12.De Falco F., Di Pace E., Cocca M., Avella M., The contribution of washing processes of synthetic clothes to microplastic pollution. Sci. Rep. 9, 6633 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xu X., Hou Q., Xue Y., Jian Y., Wang L., Pollution characteristics and fate of microfibers in the wastewater from textile dyeing wastewater treatment plant. Wat. Sci. Tech. 78, 2046–2054 (2018). [DOI] [PubMed] [Google Scholar]
- 14.Dris R., Gasperi J., Mirande C., Mandin C., Guerrouache M., Langlois V., Tassin B., A first overview of textile fibers, including microplastics, in indoor and outdoor environments. Environ. Pollut. 221, 453–458 (2017). [DOI] [PubMed] [Google Scholar]
- 15.Allen S., Allen D., Phoenix V. R., Le Roux G., Jiménez P. D., Simonneau A., Binet S., Galop D., Atmospheric transport and deposition of microplastics in a remote mountain catchment. Nat. Geosci. 12, 339 (2019). [Google Scholar]
- 16.Zubris K. A. V., Richards B. K., Synthetic fibers as an indicator of land application of sludge. Environ. Pollut. 138, 201–211 (2005). [DOI] [PubMed] [Google Scholar]
- 17.Gago J., Carretero O., Filgueiras A., Viñas L., Synthetic microfibers in the marine environment: A review on their occurrence in seawater and sediments. Mar. Pollut. Bull. 127, 365–376 (2018). [DOI] [PubMed] [Google Scholar]
- 18.Cox K. D., Covernton G. A., Davies H. L., Dower J. F., Juanes F., Dudas S. E., Human consumption of microplastics. Environ. Sci. Technol. 53, 7068–7074 (2019). [DOI] [PubMed] [Google Scholar]
- 19.Bagaev A., Mizyuk A., Khatmullina L., Isachenko I., Chubarenko I., Anthropogenic fibres in the Baltic Sea water column: Field data, laboratory and numerical testing of their motion. Sci. Total Environ. 599, 560–571 (2017). [DOI] [PubMed] [Google Scholar]
- 20.Song Y. K., Hong S. H., Eo S., Jang M., Han G. M., Isobe A., Shim W. J., Horizontal and vertical distribution of microplastics in Korean coastal waters. Environ. Sci. Technol. 52, 12188–12197 (2018). [DOI] [PubMed] [Google Scholar]
- 21.Barrows A. P. W., Cathey S. E., Petersen C. W., Marine environment microfiber contamination: Global patterns and the diversity of microparticle origins. Environ. Pollut. 237, 275–284 (2018). [DOI] [PubMed] [Google Scholar]
- 22.Obbard R. W., Sadri S., Wong Y. Q., Khitun A. A., Baker I., Thompson R. C., Global warming releases microplastic legacy frozen in Arctic Sea ice. Earths Future 2, 315–320 (2014). [Google Scholar]
- 23.Woodall L. C., Sanchez-Vidal A., Canals M., Paterson G. L., Coppock R., Sleight V., Calafat A., Rogers A. D., Narayanaswamy B. E., Thompson R. C., The deep sea is a major sink for microplastic debris. R. Soc. Open Sci. 1, 140317 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sanchez-Vidal A., Thompson R. C., Canals M., de Haan W. P., The imprint of microfibres in southern European deep seas. PLOS ONE 13, e0207033 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.de Villiers S., Quantification of microfibre levels in South Africa’s beach sediments, and evaluation of spatial and temporal variability from 2016 to 2017. Mar. Pollut. Bull. 135, 481–489 (2018). [DOI] [PubMed] [Google Scholar]
- 26.Miller R. Z., Watts A. J., Winslow B. O., Galloway T. S., Barrows A. P. W., Mountains to the sea: River study of plastic and non-plastic microfiber pollution in the northeast USA. Mar. Pollut. Bull. 124, 245–251 (2017). [DOI] [PubMed] [Google Scholar]
- 27.Dris R., Gasperi J., Rocher V., Tassin B., Synthetic and non-synthetic anthropogenic fibers in a river under the impact of Paris Megacity: Sampling methodological aspects and flux estimations. Sci. Total Environ. 618, 157–164 (2018). [DOI] [PubMed] [Google Scholar]
- 28.Kosuth M., Mason S. A., Wattenberg E. V., Anthropogenic contamination of tap water, beer, and sea salt. PLOS ONE 13, e0194970 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pauly J. L., Stegmeier S. J., Allaart H. A., Cheney R. T., Zhang P. J., Mayer A. G., Streck R. J., Inhaled cellulosic and plastic fibers found in human lung tissue. Cancer Epidemiol. Biomarkers Prev. 7, 419–428 (1998). [PubMed] [Google Scholar]
- 30.Remy F., Collard F., Gilbert B., Compoére P., Eppe G., Lepoint G., When microplastic is not plastic: The ingestion of artificial cellulose fibers by macrofauna living in seagrass macrophytodetritus. Environ. Sci. Technol. 49, 11158–11166 (2015). [DOI] [PubMed] [Google Scholar]
- 31.Taylor M. L., Gwinnett C., Robinson L. F., Woodall L. C., Plastic microfibre ingestion by deep-sea organisms. Sci. Rep. 6, 33997 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhao S., Zhu L., Li D., Microscopic anthropogenic litter in terrestrial birds from Shanghai, China: Not only plastics but also natural fibers. Sci. Total Environ. 550, 1110–1115 (2016). [DOI] [PubMed] [Google Scholar]
- 33.Watts A. J., Urbina M. A., Corr S., Lewis C., Galloway T. S., Ingestion of plastic microfibers by the crab Carcinus maenas and its effect on food consumption and energy balance. Environ. Sci. Technol. 49, 14597–14604 (2015). [DOI] [PubMed] [Google Scholar]
- 34.Jemec A., Horvat P., Kunej U., Bele M., Kržan A., Uptake and effects of microplastic textile fibers on freshwater crustacean Daphnia magna. Environ. Pollut. 219, 201–209 (2016). [DOI] [PubMed] [Google Scholar]
- 35.Song Y., Cao C., Qiu R., Hu J., Liu M., Lu S., Shi H., Raley-Susman K., He D., Uptake and adverse effects of polyethylene terephthalate microplastics fibers on terrestrial snails (Achatina fulica) after soil exposure. Environ. Pollut. 250, 447–455 (2019). [DOI] [PubMed] [Google Scholar]
- 36.Woodall L. C., Gwinnett C., Packer M., Thompson R. C., Robinson L. F., Paterson G. L., Using a forensic science approach to minimize environmental contamination and to identify microfibres in marine sediments. Sci. Rep. 95, 40–46 (2015). [DOI] [PubMed] [Google Scholar]
- 37.Torre M., Digka N., Anastasopoulou A., Tsangaris C., Mytilineou C., Anthropogenic microfibres pollution in marine biota. A new and simple methodology to minimize airborne contamination. Mar. Pollut. Bull. 113, 55–61 (2016). [DOI] [PubMed] [Google Scholar]
- 38.Primpke S., Dias P. A., Gerdts G., Automated identification and quantification of microfibres and microplastics. Anal. Methods 11, 2138–2147 (2019). [Google Scholar]
- 39.Stanton T., Johnson M., Nathanail P., MacNaughtan W., Gomes R. L., Freshwater and airborne textile fibre populations are dominated by ‘natural’, not microplastic, fibres. Sci. Total Environ. 666, 377–389 (2019). [DOI] [PubMed] [Google Scholar]
- 40.Wesch C., Barthel A.-K., Braun U., Klein R., Paulus M., No microplastics in benthic eelpout (Zoarces viviparus): An urgent need for spectroscopic analyses in microplastic detection. Environ. Res. 148, 36–38 (2016). [DOI] [PubMed] [Google Scholar]
- 41.Comnea-Stancu I. R., Wieland K., Ramer G., Schwaighofer A., Lendl B., On the identification of rayon/viscose as a major fraction of microplastics in the marine environment: Discrimination between natural and manmade cellulosic fibers using Fourier transform infrared spectroscopy. Appl. Spectrosc. 71, 939–950 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Käppler A., Fischer M., Scholz-Böttcher B. M., Oberbeckmann S., Labrenz M., Fischer D., Eichhorn K.-J., Voit B., Comparison of μ-ATR-FTIR spectroscopy and py-GCMS as identification tools for microplastic particles and fibers isolated from river sediments. Anal. Bioanal. Chem. 410, 5313–5327 (2018). [DOI] [PubMed] [Google Scholar]
- 43.Peets P., Leito I., Pelt J., Vahur S., Identification and classification of textile fibres using ATR-FT-IR spectroscopy with chemometric methods. Spectrochim. Acta A 173, 175–181 (2017). [DOI] [PubMed] [Google Scholar]
- 44.Cai H., Du F., Li L., Li B., Li J., Shi H., A practical approach based on FT-IR spectroscopy for identification of semi-synthetic and natural celluloses in microplastic investigation. Sci. Total Environ. 669, 692–701 (2019). [DOI] [PubMed] [Google Scholar]
- 45.Li L., Frey M., Browning K. J., Biodegradability study on cotton and polyester fabrics. J. Eng. Fiber Fabr. 5, 10.1177/155892501000500406 (2010). [Google Scholar]
- 46.Stark M., Letter to the editor regarding “Are We Speaking the Same Language? recommendations for a definition and categorization framework for plastic debris”. Environ. Sci. Technol. 53, 4677–4677 (2019). [DOI] [PubMed] [Google Scholar]
- 47.Park C. H., Kang Y. K., Im S. S., Biodegradability of cellulose fabrics. J. Appl. Polym. Sci. 94, 248–253 (2004). [Google Scholar]
- 48.Suaria G., Avio C. G., Mineo A., Lattin G. L., Magaldi M. G., Belmonte G., Moore C. J., Regoli F., Aliani S., The Mediterranean Plastic Soup: Synthetic polymers in Mediterranean surface waters. Sci. Rep. 6, 37551 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Arshad K., Skrifvars M., Vivod V., Valh J., Voncina B., Biodegradation of natural textile materials in soil. Tekstilec 57, 118–132 (2014). [Google Scholar]
- 50.Henry B., Laitala K., Klepp I. G., Microfibres from apparel and home textiles: Prospects for in- cluding microplastics in environmental sustainability assessment. Sci. Total Environ. 652, 483–494 (2019). [DOI] [PubMed] [Google Scholar]
- 51.Chen R., Jakes K. A., Cellulolytic biodegradation of cotton fibers from a deep-ocean environment. J. Am. Inst. Conserv. 40, 91–103 (2001). [Google Scholar]
- 52.Ryan P. G., Suaria G., Perold V., Pierucci A., Bornman T. G., Aliani S., Sampling microfibres at the sea surface: The effects of mesh size, filtered volume and water depth. Environ. Pollut. 113413 (2019). [DOI] [PubMed] [Google Scholar]
- 53.van Sebille E., Wilcox C., Lebreton L., Maximenko N., Hardesty B. D., van Franeker J. A., Eriksen M., Siegel D., Galgani F., Law K. L., A global inventory of small floating plastic debris. Environ. Res. Lett. 10, 124006 (2015). [Google Scholar]
- 54.Belzagui F., Crespi M., Álvarez A., Gutiérrez-Bouzán C., Vilaseca M., Microplastics’ emissions: Microfibers’ detachment from textile garments. Environ. Pollut. 248, 1028–1035 (2019). [DOI] [PubMed] [Google Scholar]
- 55.J. Boucher, D. Friot, Primary Microplastics in the Oceans: A global Evaluation of Sources (Gland, Switzerland: IUCN, 2017), 43 pp.
- 56.Zhu X., Nguyen B., You J. B., Karakolis E., Sinton D., Rochman C., Identification of microfibers in the environment using multiple lines of evidence. Environ. Sci. Technol. 53, 11877–11887 (2019). [DOI] [PubMed] [Google Scholar]
- 57.Waller C. L., Griffiths H. J., Waluda C. M., Thorpe S. E., Loaiza I., Moreno B., Pacherres C. O., Hughes K. A., Microplastics in the Antarctic marine system: An emerging area of research. Sci. Total Environ. 598, 220–227 (2017). [DOI] [PubMed] [Google Scholar]
- 58.Bargagli R., Environmental contamination in Antarctic ecosystems. Sci. Total Environ. 400, 212–226 (2008). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/6/23/eaay8493/DC1


