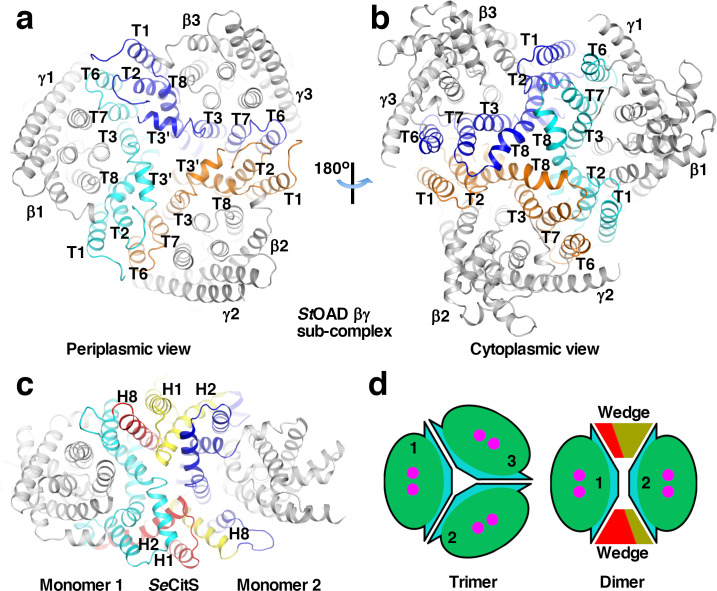
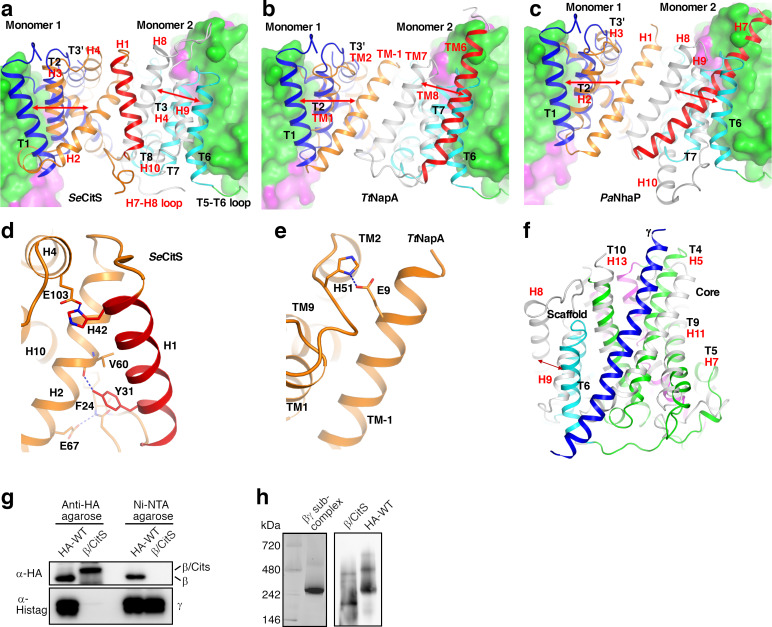
(a)-(c) Conformations of the T1 and T6 equivalents at the SeCitS (a) TtNapA (b) and the Pyrococcus abyssi NhaP (PDB 4CZ9, PaNhaP, c) dimer interfaces. The scaffold domains of monomers 1 and 2 in SeCitS, TtNapA and PaNhaP are colored in orange and gray, respectively. The extra helices in their scaffold domains are colored in red. Two StOAD β subunits are superimposed on the SeCitS, TtNapA or PaNhaP monomers. Their core domains are presented in surface representation. Their scaffold domains are presented in carton representation in blue and cyan, respectively. The red arrows indicate the large conformational differences between T1, T6 and their equivalents in SeCitS, TtNapA or PaNhaP. Red labels are for SeCitS, TtNapA or PaNhaP. In SeCitS, the T5-T6 loop equivalent (H7-H8 loop) is about 10 residues longer and allows the T6 equivalent (H8) to adopt a conformation away from the core domain. The H8 conformation is also stabilized by mostly hydrophobic interactions with the neighboring H9, and with helices H4 and H10. In TtNapA and PaNhaP, the extra helix in the scaffold domain occupies the position of T6 and ‘pushes’ the T6 equivalent away from the core domain. The conformation of the T6 equivalent is also stabilized by interactions with the T7 equivalent. (d) Hydrogen bonding and salt bridge interactions stabilizing the T1 equivalent in SeCitS (H2). (e) A salt bridge to the N-terminus of the T1 equivalent in TtNapA (TM-1) stabilizes it. (f) T6 mediates interactions between the StOAD β and γ subunits. The structure of the SeCitS monomer (gray) is superimposed. The red arrow indicates the conformational difference between T6 and the equivalent H8 in SeCitS. (g) Co-purification experiments probing the interaction between the StOAD β and γ subunits. In the β/CitS protein, T1, T6, T7 and the T5-T6 loop were substituted with equivalent regions in SeCitS, SeCitS residues N-terminal to the T1 equivalent were also included. The StOAD complex with hemagglutinin (HA)-tagged wild type β subunit or the β/CitS variant and 6x Histidine-tagged γ subunit was purified by anti-HA agarose or Ni-NTA agarose and analyzed for the β and γ subunits with anti-HA and anti-6x Histidine tag antibodies, respectively. (h) Blue native PAGE analysis probing the molecular weight of StOAD and its variants. The left and right panels were from the same gel. In the right panel, samples on the corresponding gel portion were transferred to a polyvinylidene fluoride membrane and probed with anti-HA anti-body to detect the β subunit. In the left panel, the apparent molecular weight of the major species in the Ni-NTA-purified StOAD βγ sub-complex sample (~300 kDa) is somewhat larger than the expected molecular weight of the β3γ3 hetero-hexamer (168 kDa), but most likely represent the hexamer since our EM studies indicated that it is the major species in this sample. The minor species with an apparent molecular weight of ~500 kDa might represent a dimer of the β3γ3 hetero-hexamer. In the right panel, the anti-HA agarose-purified wild type StOAD also contains a major species with an apparent molecular weight of ~300 kDa, suggesting that the β3γ3 hetero-hexamer is the major species in this sample. Since the β/CitS protein does not interact with the γ subunit that mediate interactions with the α subunit (panel g), the anti-HA agarose-purified StOAD with the β/CitS substitution should contain only the β/CitS protein. Two species were observed for this sample (right panel). The apparent molecular weight of the lower molecular weight species is significantly smaller than that of the β3γ3 hetero-hexamer. The size of the β/CitS monomer (53 kDa) is close to the size of the β and γ monomers combined (57 kDa including the HA tag), suggesting that the low molecular weight species is unlikely a β/CitS trimer but a dimer. The high molecular weight species may represent a dimer of the β/CitS dimer.