Fig. 1. Factors controlling the short-pitch transition in recombinant human Arp2/3 complex.
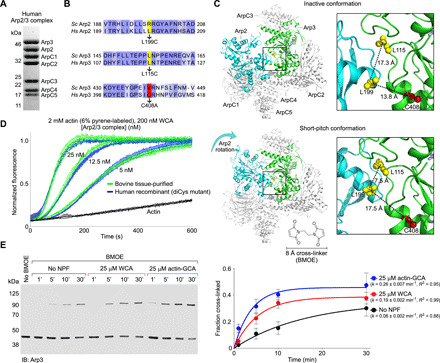
(A) SDS-PAGE of recombinantly expressed human Arp2/3 complex. (B) Sequence alignment of human and budding yeast Arp2 and Arp3, showing the three amino acids mutated to generate the diCys mutant used in the cross-linking assay by analogy with the yeast complex (29). (C) Illustration of the cross-linking assay; Arp2 L199 and Arp3 L115 (both mutated to cysteine residues) are within BMOE cross-linking distance (~8 Å) only in the short-pitch conformation, in which Arp2 is moved alongside Arp3, analogous to two adjacent subunits of the actin filament. The C408A mutation in Arp3 was introduced to prevent spurious cross-linking. (D) Time course of actin polymerization by diCys Arp2/3 complex mutant and tissue-purified bovine Arp2/3 complex over a range of concentrations (n = 3, per concentration). (E) Western blot analysis (immunoblotting: Arp3) and densitometric quantification (n = 3) of the cross-linked fraction of diCys Arp2/3 complex in the absence of NPFs and with either WCA or actin-GCA and different BMOE treatment times (as indicated). Data were fit to a first-order exponential function to obtain the kinetic parameters.
