Fig. 4. Interaction of the C helix with the Arps and comparison with related helices from other domains.
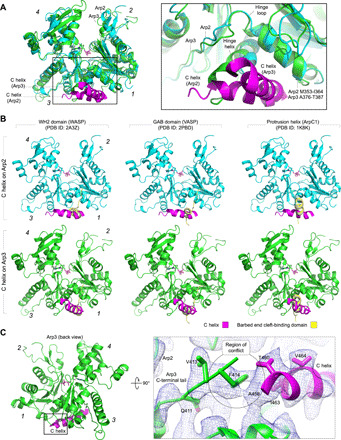
(A) Superimposition of Arp2 (cyan) and Arp3 (green), showing how the orientation of the C helix (magenta) of NPFs is different for the two Arps (numbers in italic designate subdomains 1 to 4). The inset shows that the hinge helix is one helical turn longer in Arp3 than in Arp2, which would create a clash with the C helix if it were to bind to Arp3 the way it binds to Arp2. (B) The interaction of the C helix with Arp2 (top row) and Arp3 (bottom row) differs from those of the WH2 domain (left column, yellow) and GAB domain (middle column, yellow) with actin (blue) and from the crystal contact made by the protrusion helix of ArpC1 with Arp3 in the structure of inactive Arp2/3 complex (right column, yellow). (C) The N-terminal end of the C helix on Arp3 clashes with the C-terminal tail of Arp3, which would have to move for the C helix to bind. The inset (right) shows a close-up view of this clash, with the cryo-EM map contoured at 6.6 σ.
