Figure 3.
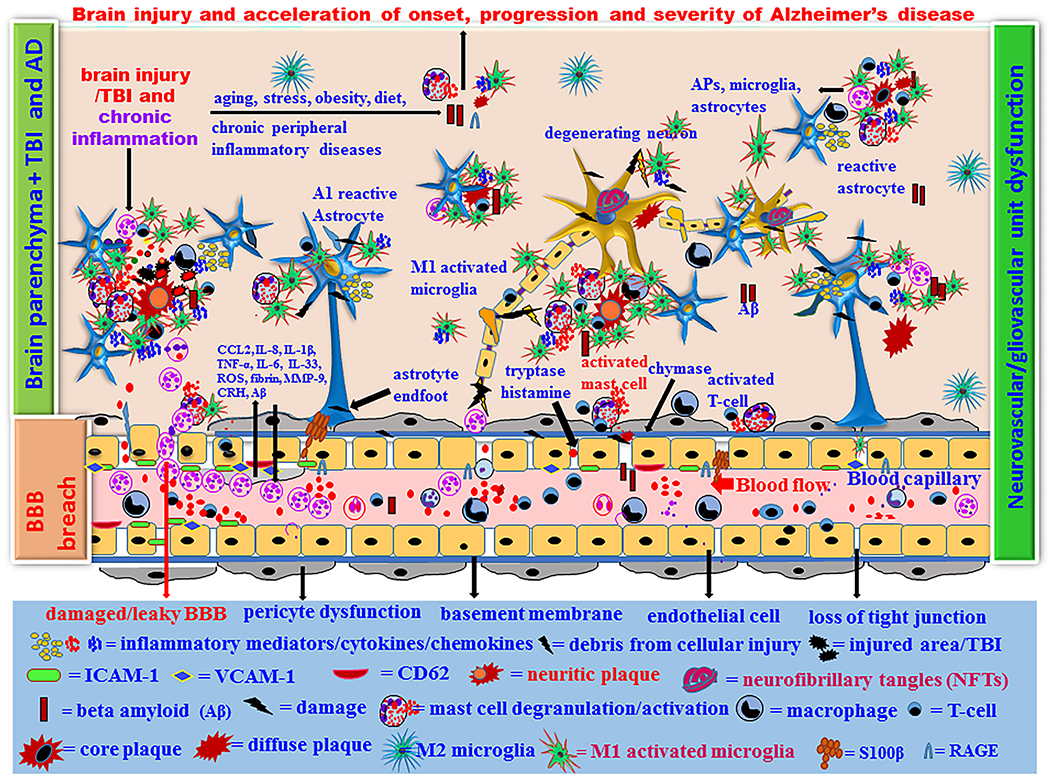
Proposed brain injury/TBI-associated onset, progression and severity of AD in high-risk people. Brain injury leads to microbleeds and acute primary damage of brain cells. If acute immune response continues chronically, that leads to secondary damage of brain cells due to over activation of immune cells associated with s significantly higher levels of inflammatory and neurotoxic mediator release in a vicious fashion in the brain and peripheral immune system. Increased number of neurotoxic and inflammatory M1 microglia and A1 astrocytes exacerbates neuroinflammation and neurodegeneration in the injured brain. Normal aging process and brain injury generate APP and Aβ1–42 in the brain and these chronically activate neuroglia and immune cells in the brain that release several neuroinflammatory and neurotoxic mediators. Aβ and tau protein generate the formation of extracellular APs, and intracellular NFTs in the neurons, respectively. Immune system fails to remove the excess Aβ peptide and NFTs and thus the levels of these increases continuously. This is associated with the degeneration of pericytes and endothelial cells, increased neuroinflammation and neurodegeneration in the brain. This ultimately cause cognitive dysfunction and the onset of AD. Brain injury/TBI in high-risk people with PD, anxiety, depression, PTSD, CTE, ALS, aging, obesity, emotion, chronic peripheral diseases, chronic allergic tendencies, genetic factors, environmental factors, and high fat diet can accelerate the pathogenesis of AD.
