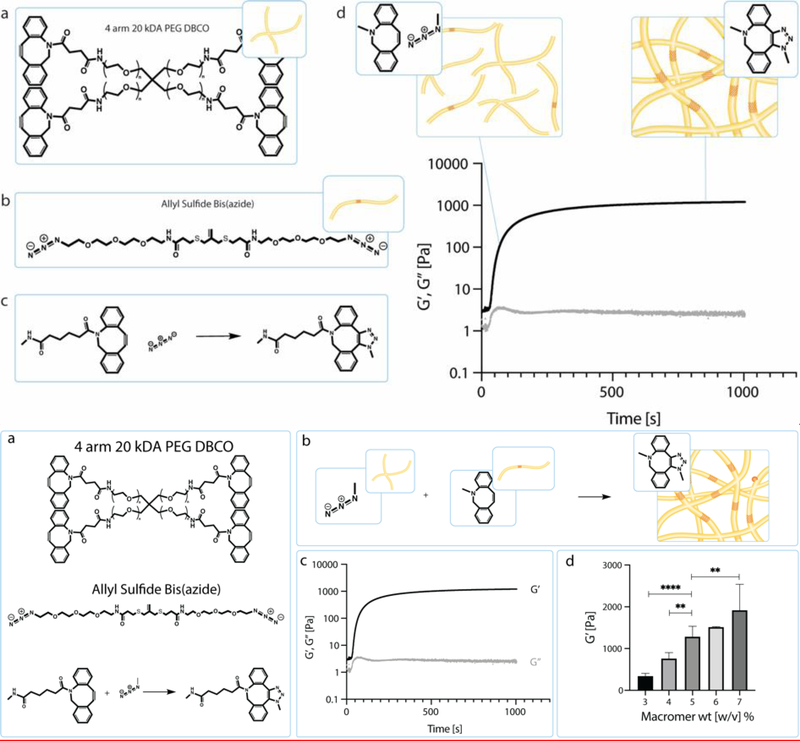
Figure 1.
a) Schematic of the chemical structure of 4 arm 20 kDa PEG DBCO and the chemical structure of allyl sulfide bis(azide). Upon mixing the PEG DBCO with the allyl sulfide bis(azide), the strained octyne will react with the azide group through a strain-promoted azide alkyne cycloaddition (SPAAC). b) Through this SPAAC formation, a hydrogel network is formed. The red cross hatch region of the network is the photodegradable region. c) Shear storage modulus (black) and loss modulus (gray) were measured over time as the network polymerizes. Polymerization is rapid and occurs in under 5 min. d) Hydrogel moduli increase with increasing macromer wt%. Data is represented as means +/− standard deviation. An n of 3 was used and analyzed using a one-way Anova with multiple comparisons. **p<0.01, ****p<0.0001