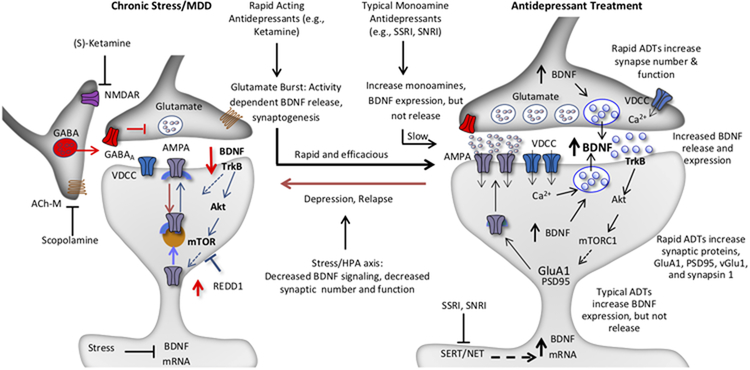
Figure 2. Model for the loss of spine synapses in stress and depression: rapid reversal by ketamine and comparison with typical monoamine reuptake inhibitor antidepressants.
Synaptic number and function in the PFC and hippocampus are maintained by homeostatic mechanisms, and proper control of synaptic connectivity in these regions is required for control of mood as well as other cortical functions. This includes activity dependent regulation of synaptic proteins, such as GluA1, PSD95, and synapsin 1. Chronic stress exposure (left) decreases the number and function of spine synapses in the PFC and hippocampus, in part via decreased BDNF expression, and decreased TrkB-mTORC1 signaling. Increased expression of a negative regulator of mTORC1 signaling, regulated in DNA damage and repair (REDD1) contributes to this effect. Antidepressant treatment (right) increases BDNF and reverses the effects of stress and depression. The NMDA receptor antagonist ketamine causes a rapid burst of glutamate that causes activity dependent release of BDNF resulting in rapid induction of synapse number and function via stimulation of TrkB-mTORC1 signaling and increased translation of synaptic proteins including GluA1. In contrast, typical antidepressants including the monoamine reuptake inhibitors (e.g., SSRI, SNRI) produce a slow induction of BDNF expression and constitutive release over several weeks of treatment, which contributes to slow adaptive changes that reverse the effects of stress and produce antidepressant responses. There is no evidence that typical antidepressants cause activity dependent release of BDNF, which is necessary for the rapid antidepressant actions of ketamine. Depression as well as relapse that occurs after 7 to 10 days in patients treated with ketamine could result from environmental factors such as sustained or uncontrollable stress or other susceptibility factors that decrease the stability of new synaptic connections.