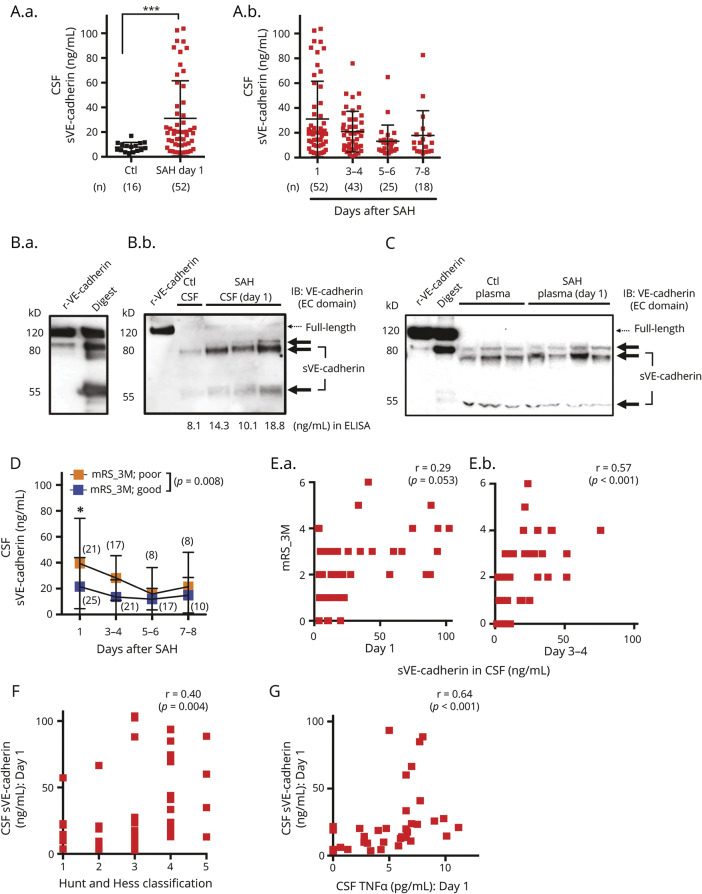
Figure 1. Soluble vascular endothelial (sVE)-cadherin is present at higher levels in the CSF of patients with subarachnoid hemorrhage (SAH) and is associated with functional outcome.
(A.a.) CSF sVE-cadherin level in patients with SAH on day 1 after SAH compared to that of controls (p = 0.0002, Mann-Whitney U test). (A.b.) CSF sVE-cadherin levels on subsequent days after SAH. No control samples are available on these days. (B.a.) 80 and 55 kD proteins are the predominant forms of sVE-cadherin fragments generated when recombinant human vascular endothelial (VE)-cadherin is incubated with recombinant human ADAM10 (digest). (B.b.) The major forms of VE-cadherin in the CSF of control patients and patients with SAH are also 80 and 55 kD. CSF with higher VE-cadherin levels measured by ELISA contains the same fragments in larger quantities, with minimal amounts of full-length VE-cadherin. (C) A representative Western blot of plasma shows that the major forms of VE-cadherin in the plasma of control patients and patients with SAH are comprised of 80 and 55 kD fragments with minimal amounts of full-length VE-cadherin. (D) Patients with SAH who developed poor outcome (modified Rankin Scale [mRS] > 2) at 3 months had higher CSF sVE-cadherin levels over time compared to those who had good outcome (mRS < 2) at 3 months (p = 0.008 using mixed model analysis with autoregressive covariance structure and with log-transformation for non-normally distributed data). (E) There is a positive correlation between mRS-3M and CSF sVE-cadherin levels collected on day 1 (E.a.; r = 0.29, p = 0.053) and day 3-4 (E.b.; r = 0.57, p < 0.001; Spearman correlation). (F) Initial Hunt and Hess classification and CSF sVE-cadherin level are positively correlated on day 1 after SAH (Spearman correlation, r = 0.40, p = 0.004). (G) CSF TNF- α and CSF sVE-cadherin levels are positively correlated on day 1 (r = 0.64, p < 0.001; Spearman correlation). EC = extracellular; IB = immunoblot; r-VE-cadherin = recombinant human vascular endothelial-cadherin.