There is increasing evidence that severe acute respiratory syndrome coronavirus (CoV) 2 (SARS-CoV-2) impacts the neurologic system.1, 2, 3 However, how the immunosuppressed state modifies neurologic involvement and clinical course remains uncertain.4 We describe a patient with heart transplant who developed prolonged symptoms of encephalopathy late in CoV disease 2019 (COVID-19) illness.
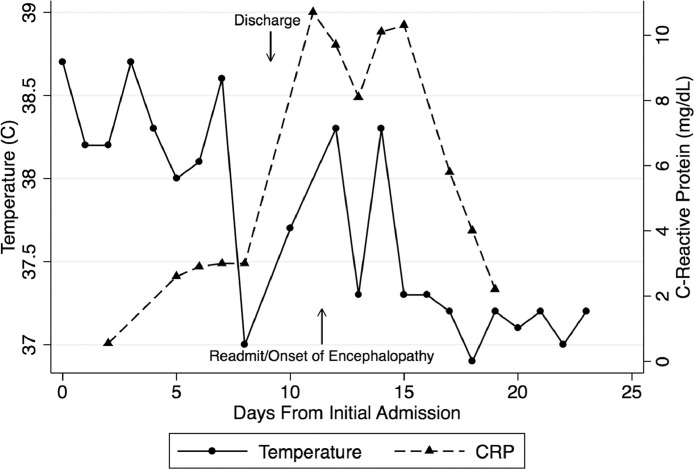
A 67-year-old man was admitted with fever, cough, nasal congestion, sore throat, and diarrhea 20 months after an uneventful heart transplantation. He was not hypoxic. Chest computed tomography (CT) showed bilateral multifocal peripheral ground-glass opacities (GGOs). White blood cell count was 4,720/µl with lymphocyte count of 1,040/µl. Blood cultures and cytomegalovirus polymerase chain reaction (PCR) were unremarkable. C-reactive protein (CRP) was 0.55 mg/dl. Nasopharyngeal PCR for SARS-CoV-2 was positive, consistent with COVID-19 illness. Immunosuppression included 500 mg mycophenolate twice daily (BID) and tacrolimus with trough level of 7.6 ng/ml at admission. Mycophenolate was discontinued. Tacrolimus was maintained for a goal level of 7‒10 ng/ml. He continued to have intermittent fevers (Figure 1 ) but was never hypoxic. Chest CT on Day 6 showed worsening GGOs. Hydroxychloroquine was started on Day 7 (400 mg BID on the first day, then 200 mg BID for 4 days). He was discharged on Day 9.
Figure 1.
Fever and CRP Trend Following Admission. CRP, C-reactive protein.
He was readmitted after 2 days with confusion, anorexia, and emesis. He was alert, oriented only to person, and febrile. He had no previous history of delirium or encephalopathy and was on stable home dose of venlafaxine for the treatment of a history of depression. Examination was notable for postural and action tremor, without focal neurologic deficits. CRP was increased to 10.7 mg/dl with a mild elevation in aspartate transaminase and alanine transaminase to a maximum of 74 U/liter and 79 U/liter, respectively. Serum ammonia and thyroid-stimulating hormone were normal. Serum sodium was decreased to 133 milliequivalents/liter (normal range 135–145 milliequivalents/liter) but returned to normal by Day 2 of readmission. Blood urea nitrogen and serum creatinine were 42 mg/dl and 1.73 mg/dl, respectively, unchanged from baseline. Trough tacrolimus level at readmission was 11.5 ng/ml and averaged to 10 ± 1.2 ng/ml during the admission. Hydroxychloroquine was discontinued.
CT scan of the head was unremarkable. Chest CT showed minimal progression of GGOs. Brain magnetic resonance imaging showed mild scattered foci of peri-ventricle deep and sub-cortical white matter ischemia. There was no evidence of encephalitis, posterior reversible encephalopathy, or leukoencephalopathy. Repeat magnetic resonance imaging after 1 week was unchanged. Electroencephalogram showed mild non-specific diffuse multifocal cerebral dysfunction with no seizure seen. Lumbar puncture showed 1 lymphocyte, normal protein, and glucose. Cerebrospinal fluid (CSF) was negative for herpes simplex PCR and Cryptococcus antigen. PCR of CSF, not validated for the detection of SARS-CoV-2, was negative. By Day 16, CRP began to decline. Mental status slowly improved, and he was discharged 13 days after readmission. Executive function and memory remained poor but gradually returned to normal approximately 45 days after the onset of the encephalopathy.
Whereas we were unable to demonstrate the presence of SARS-CoV-2 virus in the CSF of our patient, no alternative diagnosis for his encephalopathy was found. Sepsis was unlikely to be an explanation because symptoms started after clinical stabilization and he had limited signs or symptoms of organ dysfunction. Hydroxychloroquine has been associated with neurologic changes, but symptoms persisted for more than 2 weeks after discontinuation.5 The onset of encephalopathy coincided with increased markers of inflammation, most notably CRP. The impact of immunosuppression in patients with COVID-19 remains uncertain, and the severity of illness in case reports has been varied.6 , 7 It is possible that immunosuppression may attenuate the severity of disease by limiting the extent of cytokine release, which may be the primary mediator of injury8; however, a recent report suggests poor outcomes in hospitalized patients with solid organ transplant.9 The optimal degree of immunosuppression in patients with heart transplant with COVID-19 remains uncertain.
COVID-19 has been associated with acute hemorrhagic necrotizing and hypoxic encephalopathy, encephalitis, delirium, and anosmia.1 , 10, 11, 12 Other β-CoVs such as severe acute respiratory syndrome-CoV-1 and Middle East respiratory syndrome-CoV have been shown to be neurotropic and manifest with delirium.1 The angiotensin-converting enzyme 2 receptor (the primary source of cell entry of the virus) is expressed in both neurons and glia and could serve as a mechanism of direct viral entry into the nervous system. Alternatively, the inflammatory state itself may give rise to encephalopathy,13 and patients with acute respiratory distress syndrome have been shown to have a high rate of persistent dysexecutive syndrome similar to that of our patient3; however, our patient was never hypoxic or severely ill before the onset of symptoms, and symptoms persisted for many days after normalization of markers of inflammation. Given the novel presentations of COVID-19, we believe it is important for transplant centers to consider the potential for delayed neurologic manifestations.
References
- 1.Steardo L, Steardo L Jr, Zorec R, Verkhratsky A. Neuroinfection may contribute to pathophysiology and clinical manifestations of COVID-19 [e-pub ahead of print]. Acta Physiol (Oxf) doi:10.1111/apha.13473, accessed March 26, 2020. [DOI] [PMC free article] [PubMed]
- 2.Wu Y, Xu X, Chen Z, et al. Nervous system involvement after infection with COVID-19 and other coronaviruses [e-pub ahead of print]. Brain Behav Immun doi:10.1016/j.bbi.2020.03.031, accessed March 30, 2020. [DOI] [PMC free article] [PubMed]
- 3.Helms J, Kremer S, Merdji H. Neurologic features in severe SARS-CoV-2 infection. N Engl J Med. 2020;382:2268–2270. doi: 10.1056/NEJMc2008597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fishman JA, Grossi PA. Novel coronavirus-19 (COVID-19) in the immunocompromised transplant recipient: #flatteningthecurve [e-pub ahead of print]. Am J Transplant doi:10.1111/ajt.15890, accessed April 4, 2020. [DOI] [PMC free article] [PubMed]
- 5.Mittal L, Zhang L, Feng R, Werth VP. Antimalarial drug toxicities in patients with cutaneous lupus and dermatomyositis: a retrospective cohort study. J Am Acad Dermatol. 2018;78:100–106.e1. doi: 10.1016/j.jaad.2017.09.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guillen E, Pineiro GJ, Revuelta I, et al. Case report of COVID-19 in a kidney transplant recipient: does immunosuppression alter the clinical presentation [e-pub ahead of print]?Am J Transplant doi:10.1111/ajt.15874, accessed March 26, 2020. [DOI] [PMC free article] [PubMed]
- 7.Li F, Cai J, Dong N. First cases of COVID-19 in heart transplantation from China. J Heart Lung Transplant. 2020;39:496–497. doi: 10.1016/j.healun.2020.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mehta P, McAuley DF, Brown M. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395:1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pereira MR, Mohan S, Cohen DJ, et al. COVID-19 in solid organ transplant recipients: initial report from the US epicenter [e-pub ahead of print]. Am J Transplant doi:10.1111/ajt.15941, accessed April 24, 2020. [DOI] [PMC free article] [PubMed]
- 10.Poyiadji N, Shahin G, Noujaim D, Stone M, Patel S, Griffith B. COVID-19-associated acute hemorrhagic necrotizing encephalopathy: CT and MRI features [e-pub ahead of print]. Radiology doi:10.1148/radiol.2020201187, accessed March 30, 2020. [DOI] [PMC free article] [PubMed]
- 11.Chen T, Wu D, Chen H. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ. 2020;368:m1091. doi: 10.1136/bmj.m1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ye M, Ren Y, Lv T. Encephalitis as a clinical manifestation of COVID-19 [e-pub ahead of print]. Brain Behav Immun doi:10.1016/j.bbi.2020.04.017, accessed April 10, 2020. [DOI] [PMC free article] [PubMed]
- 13.Troyer EA, Kohn JN, Hong S. Are we facing a crashing wave of neuropsychiatric sequelae of COVID-19? Neuropsychiatric symptoms and potential immunologic mechanisms [e-pub ahead of print]. Brain Behav Immun doi:10.1016/j.bbi.2020.04.027, accessed April 13, 2020. [DOI] [PMC free article] [PubMed]