Abstract
Background
Peripartum cardiomyopathy (PPCM) is life-threatening heart disease. However, the causes and pathogenesis of PPCM remain unclear. Previous studies found that β1 adrenoceptor antibodies (β1AA) had possible involvement in the development of PPCM. In the present study, we determined the potential relationship between PPCM and β1AA, including the mechanism of β1AA leading to PPCM.
Methods
We extracted the β1AA from the postpartum Wistar rats that were injected by the antigen peptide segment of the β1 adrenoceptor to produce PPCM. We tested the effects of β1AA on H9C2 cell line by CCK-8, LDH, TUNEL, SA-ELISA, qRT-PCR, and western blot methods. Furthermore, PGC-1α was overexpressed to rescue the effect of β1AA on H9C2 cells.
Results
We found that the extracted β1AA induced apoptosis of cardiac myocytes of H9C2 cell line. Moreover, the expression of peroxisome proliferator-activated receptor γ coactivator-1α (PGC-1α), which is a master regulator of mitochondrial metabolism, and its downstream transcript vascular endothelial growth factor (VEGF) got decreased in H9C2 cells after β1AA treatment. In addition, the effect of β1AA could be inhibited by atenolol, the antagonist of β1 adrenoceptors (β1AR) and imitated by isoprenaline, the agonist of β1AR. Furthermore, overexpression of PGC-1α in the H9C2 cells rescued the apoptosis of cells and inhibitory expression of VEGF induced by β1AA.
Conclusions
Our results suggest that the symptoms of PPCM due to myocardial cell apoptosis induced by β1AA inhibiting the PGC-1α-related pathway impairs mitochondrial energy metabolism. Therefore, our results uncover a previously unknown role of the β1AA pathway in the etiology of PPCM and provide a novel potential target for the treatment of PPCM.
Keywords: Peripartum cardiomyopathy, Autoantibody against β1 adrenoceptor, Peroxisome proliferator-activated receptor γ coactivator-1α, Vascular endothelial growth factor, Apoptosis
Background
β1 adrenoceptor (β1AR) plays an important role in adrenergic regulation of myocardial contractility, which induces positive chronotropic, inotropic, and dromotropic action on myocardial through stimulatory Gs protein [1]. However, from the past two decades, researchers found that the β1-adrenoceptor antibodies (β1AA) participate in the dilated cardiomyopathy (DCM). Peripartum cardiomyopathy (PPCM) is one kind of DCM. The pregnant women who are in good health seem to have cardiac failure for unknown reasons [2]. Although patients with PPCM have no prior history of heart disease and there are no other known possible causes of heart failure, our previous study found that there was a high correlation between β1AA and the development of PPCM [3]. However, the mechanisms involved in β1AA inducing PPCM are not fully understood.
PPCM, defined as idiopathic cardiomyopathy, represents heart failure secondary to left ventricular systolic dysfunction towards the end of pregnancy or in the months following delivery [4]. It was found that inappropriate signaling and metabolic derangement at the level of the mitochondrion might be one of the key reason leading to myocardial hypertrophy [5, 6]. In addition, mitochondria play a major role in apoptosis, which is greatly associated with heart failure [7]. Reports state that β1AA induce apoptosis in cardio myocytes isolated from normal rats [8], suggesting the mechanism of PPCM induced by β1AA may relate to mitochondrial dysfunction.
Peroxisome proliferator-activated receptor γ coactivator-1α (PGC-1α) is a key integrator of transcriptional circuits regulating mitochondrial biogenesis and function [9]. Activation of the PGC-1α regulatory cascade increases cardiac mitochondrial oxidative capacity in the heart [9] and protects the cells from apoptosis [10]. To test the hypothesis that β1AA inducing PPCM relates to mitochondrial dysfunction, initially, we developed immune PPCM models by injecting the antigenic peptide segment of β1AR into postpartum Wistar rats and extracted the autoimmune β1AA from these animals. Later, we examined the effects of extractive β1AA on survival and apoptosis in myocardial H9C2 cells and the expression of β1AR, caspase3, PGC-1α, and vascular endothelial growth factor (VEGF), which is the downstream transcript of PGC-1α. Finally, we overexpressed the PGC-1α in H9C2 cells to observe whether it can rescue the cell’s apoptosis after β1AA treatment.
Methods
Study design
Previous study found that there was a high correlation between β1AA and development of PPCM [3], indicating a possible involvement of β1AA in the development of PPCM. In the present study, β1AA were extracted from the postpartum Wistar rats, which were injected the antigen peptide segment of the β1 adrenoceptor to produce autoimmunity and the effects of extracted β1AA on the H9C2 cells were examined.
Animals and active immunity
Ten postpartum Wistar rats were obtained from Model Animal Research Center of Nanjing University, maintained in specific pathogen-free (SPF) conditions under a 12 h-light-12 h-dark cycle. All animal experiments were approved by the Institutional Animal Care and Use Committee (IACUC) of Nanjing University, Nanjing, China, and was conducted in accordance with the Guide for the Care and Use of Laboratory Animals from the National Institutes of Health (Bethesda, MD, USA). Animals were randomly divided into two groups, and five in each group. The antigenic peptide segment of β1 adrenoceptor was purchased from GL Biochem company (Shanghai, China). For first immunization, the antigenic peptide segment was dissolved into the Na2CO3 solution, and mixed the solution with freund’s incomplete adjuvant 1: 1. The final concentration of antigenic peptide segment in the mixture was 1 mg/ml. Then the mixture was injected subcutaneously in back of each animal with antigenic peptide segment 0.4 μg/g. For the negative control, Na2CO3 solution was injected with the same protocol. Two weeks after the first immunization, immune process was repeated again. Intravenous blood samples were performed on each groups in the third week. After animal experiments, the animals were euthanized with intraperitoneal injection of excessive pentobarbital (100-150 mg/kg).
Autoimmune β1AA procedure extraction
The affinity chromatography method was employed to extract proteins from serum. Four grams of the Sepharose4B (Sigma, Aldrich, USA) were immobilised onto 30 ml Sepharose column according to the manufacturer’s instructions (Amersham, Uppsala, Sweden). Three millilitres of conditioned or control serum were 1:8 diluted in PBS respectively, and applied onto the coupled-column at room temperature. After extensive washing with PBS until the value of OD280 < 0.05, the bound proteins were eluted with 3 M MgCl2. The eluted proteins were collected for each IgG according to the value of OD280 and dialyzed against PBS. The eluted proteins were analyzed by 12% SDS/PAGE under reducing conditions. In addition, quantification of the eluted proteins was determined by BCA (bicinchonininc acid) assay.
Cells culture and transfection experiments
H9C2 cells were purchased from cell bank of the Chinese Academy of Sciences (Shanghai, China, Cat#: CNR 5) and cultured in F12-DMEM medium with 10% fetal bovine serum. All these cells were incubated at 37 °C, 5% CO2. The medium was changed for 2 days and the cells were subcultured. One day before experiments, the cultured cells were digested with 0.25% trypsin + 0.02% EDTA. After digestion, cells were triturated to free collected by centrifugation (1500 rpm, 5 min). Then, cells were seeded in a 96-well plate at a density of 1 × 104 cells per well and incubated at 37 °C, 5% CO2 over night. After these treatments, 1 μM β1AA, 1 μM nonspecific IgG, 1 μM isoprenaline or mixture of 1 μM β1AA and 1 μM atenolol were added into the cell-culture medium with processing 12 h. Then, subsequent experiments were carried out. For overexpression of PGC-1α, the expression plasmid pcDNA3-PGC-1α encoding PGC-1α was constructed by our laboratory and plasmid expressing shRNA for PGC-1α and its control shRNA was purchased from Thermo Scientific. Cells were transiently transfected with plasmid for 24 h before the experiments, using Lipofectamine® 2000 Transfection Reagent (Invitrogen, Carlsbad, American, Cat#: 11668–019) according to the manufacturer’s recommendations.
CCK-8 assay
Cytotoxicity of β1AA was evaluated by CCK-8 assay in H9C2 cells. The CCK-8 detection kit was purchased from 7sea Biotech company (Shanghai, China, Cat#: 20140419). Briefly, after treatments, a 10-ul of CCK-8 or F12-DMEM medium were added to each well and the cells were further incubated for 1 h and the absorbance of each well was measured using a Microplate Reader (Model 680, BIO-RAD, USA) at the wavelength of 450 nm.
LDH assay
Measurements of LDH release were performed following the manufacturer’s instructions. The LDH detection kit was purchased from Promega company (Madison, American, Cat#: G7891) After cells were exposed to β1AA or nonspecific IgG suspensions, the exposure medium was collected and centrifuged at 1500 rpm for 10 min. The supernatant was mixed with the LDH assay mixture at a ratio of 1:1 and incubated at room temperature in the dark for 30 min. The reaction was stopped by the addition of 1 N HCl (1/10 of mixture volume), and the absorbance at 590 nm. Viability in relation to the control (the unexposed group) is calculated from equation:
Viability(%Control) = (LDHlysed − LDHExposed) / (LDHlysed − LDHcontrol)(100%).
where LDHlysed is the LDH released from wells treated with the LDH lysing solution (total cellular LDH content), LDHexposed is the LDH released from wells exposed to β1AA or nonspecific IgG suspensions, and LDH control is the LDH released from cells in the control group.
Terminal deoxynucleotidyltransferase-mediated dUTP nick-end labeling (TUNEL) of myocardial cells
A fluorometric TUNEL detection kit (Genecopoeia, Rockerville, USA, Cat#: A050) was used to detect apoptotic DNA strand breaks. The H9C2 cells were fixed with 4% neutral buffered formaldehyde in PBS (pH 7.4) at 25 °C for 30 min, permeated with 50 μg/ml proteinase K at 25 °C for 15 min, and incubated with the labeling reaction mixture in a humidified chamber at 37 °C for 1 h. The cells were then processed with a standard immunocytochemical staining procedure to incubate with antibody against DAPI (a cell nucleus marker; Invitrogen, Carlsbad, CA). Finally, a leica fluorescence microscope (DM6000B, Leica, Germany) was used to capture the images, and the ratio of TUNEL positive nuclei in total (DAPI positive nuclei) was computed to express the cells apoptosis.
Sa-ELISA
To test the concentration of VEGF, SA- ELISA was performed. Precisely, the medium of cell cultures was added into a 96-well plate 100 μl per well. The wells were then incubated for 1 h at 37 °C with antibody labeled with biotin anti-rat monoclonal IgG (1:1000, 50 μL/well) (BOSTER, Cat#: BA1005). Next, streptomycin with horseradish peroxidase (1:500, 50 μL/well) (Bioss, Cat#: bs-0437P-HRP) was added into the wells and incubated for 1 h at 37 °C. Finally, chromogenic tetramethylbenzidine (TMB) solution (50 μL/well) was added into each well and incubated for 10-30 min at 37 °C. Optical density (OD) was measured at 450 nm on a micro plate reader (Erba Lisa Scan II, Germany). The OD value was compared with the standard and the final concentration was obtained.
Quantitative real time-PCR (qRT-PCR)
Total RNA of H9C2 cells was isolated using Ultrapure RNA Kit (Cat#: CW0581S, CWBio). 400 ng of RNA was subjected to reverse transcription-PCR with SuperRT cDNA Synthesis Kit (Cat#: CW0741S, CWBio) according to the instruction. Quantitative RT-PCR was performed with PCR primers listed in Table 1. UltraSYBR Mixture Kit (Cat#: CW2602M, CWBio) was employed to detect mRNA levels of these genes. All reactions were repeated 3 times and GAPDH was used to normalize target.
Table 1.
List of utilized primers for qRT-PCR
| Gene | Forward primer | Reverse primer |
|---|---|---|
| β1AR | CGACTGCTGGTGCTCGCGTCG | AGCGAAAGGGCAGCGTGATGGC |
| PGC-1α | CGCACAACTCAGCAAGTCCTC | CCTTGCTGGCCTCCAAAGTCTC |
| PGC-1β | CAAGAAGCGGCGGGAAA | GCTCATGTCACCGGAGAGATTT |
| VEGF | GCAGCGACAAGGCAGACTATT | ACCGTTGGCACGATTTAAGAG |
| NRF1 | CCACGTTGGATGAGTACACG | CTGAGCCTGGGTCATTTTGT |
| ERRα | AAGCCCTGATGGACACCTC | GAAGCCTGGGATGCTCTTG |
| GAPDH | TGGAGTCTACTGGCGTCTT | TGTCATATTTCTCGTGGTTCA |
Western blot analysis
The H9C2 cells were lysed in RIPA buffer (P0013C, Beyondtime) containing 1 mM PMSF (ST505, Beyondtime). Total protein of 30 μg was subjected to electrophorese on 12–6% SDS-Page gels and transferred to PVDF membranes. Antibodies against β1AR (1:1000; cat. bs-0498R, Bioss), VEGF (1:800; cat. AF5131, Affinity), PGC-1α (1:1000, cat. bs-1832R, Bioss), and caspase3 (1:1000; cat. bs-0081R, Bioss) were used as primary antibodies. Rabbit IgG antibodies coupled to horseradish peroxidase (HRP) were used as secondary antibodies. GAPDH (1:1000; cat. BA2913, Boster) was used as loading control. An enhanced chemiluminescence (ECL) system was used for detection of protein bands.
Statistical analysis
All data were presented as mean ± SEM. One-way ANOVA was conducted to evaluate the one-way layout data. If a significant difference was observed, Bonferroni’s post-hoc test was conducted to identify groups with significant differences. The relative mRNA levels were calculated using the 2-ΔΔCt method. All analyses were performed using SPSS 19.0. Differences were considered significant with p < 0.05.
Results
Validation of extractive β1AA
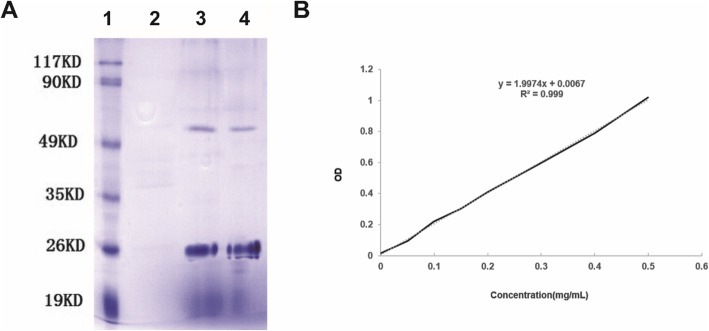
To investigate the effect of β1AA on the PPCM first, β1AA were extracted from the autoimmune postpartum Wistar rats by using the Affinity chromatography method. In Fig. 1a, the negative sera lacked bands whereas the immune group had two bands in the sera at 55 KD and 25 KD, which remained consistent with the standard products and we also tested the concentration of the β1AA in the serum where the final concentration was 3.63 mg/ml (Fig. 1b).
Fig. 1.
Validation of extractive β1AA. a SDS-PAGE gel electrophoresis raw data of serum. Lane 1, Marker; Lane 2, Negative serum; Lane 3, Serum of autoimmune postpartum animals; Lane 4, Standard sample. b Standard curve of OD-concentration. The protein concentration of Serum of autoimmune postpartum animals was 3.63 mg/kg
The effect of extractive β1AA on survival and apoptosis in myocardial H9C2 cells
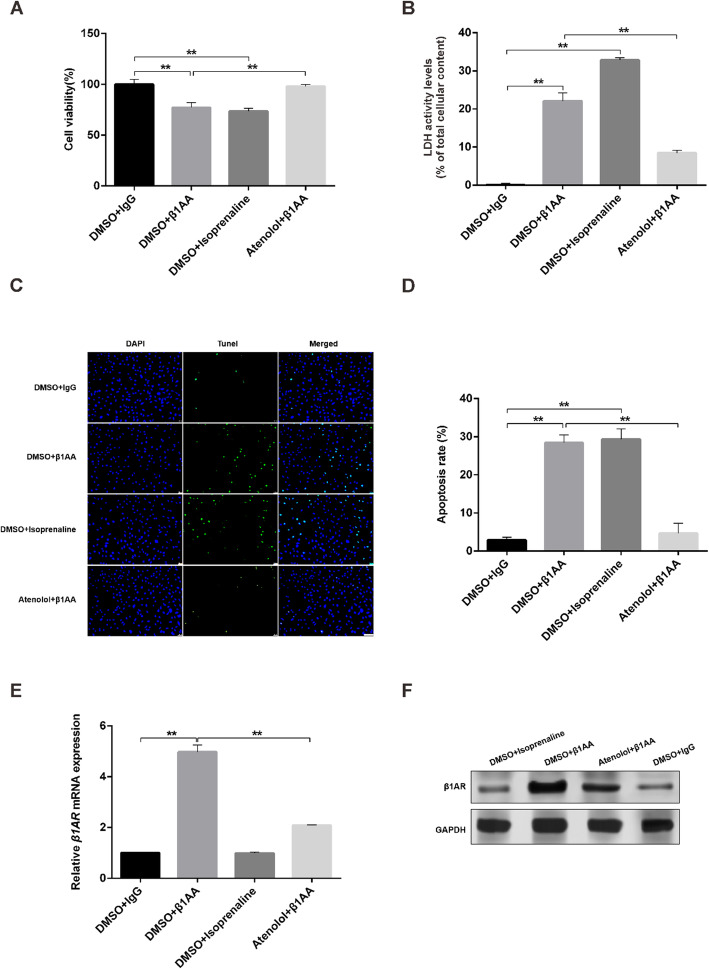
The results of the CCK8 assay (Fig. 2a) indicated that β1AA significantly inhibited cell proliferation compared with the control group. From the previous studies, we found that β1AA was a weak agonist of the β1AR; hence we used isoprenaline, the agonist of the β1AR and atenolol, the antagonist of β1AR to mimic and inhibit the effect of β1AA on inhibitory proliferation in H9C2 cells. We found that isoprenaline had the same effect as β1AA and atenolol could reverse the inhibitory effect on the proliferation of H9C2. We found similar results in LDH (Fig. 2b) and Tunel (Fig. 2c and d) assay. The apoptosis of H9C2 cells significantly increases after β1AA and isoprenaline treatment. And atenolol inhibited the effect of β1AA on apoptosis of H9C2 cells. Finally, compared to the negative IgG group, the mRNA and protein level of β1AR significantly increased after β1AA treatment and atenolol inhibited the effect of β1AA on the expression (Fig. 2e and f). However, although isoprenaline was the agonist of β1AR as β1AA, it did not affect the expression of β1AR. All above results suggested that the effect of β1AA on H9C2 cells was through β1AR.
Fig. 2.
Extractive β1AA decreased the rate of survival and increased the rate of apoptosis in myocardial H9C2 cells. a The results of CCK8 assay. β1AA significantly inhibited the cell proliferation. The effect of β1AA could be mimiced by isoprenaline, an agonist of β1AR and be inhibited by atenolol, an antagonist of β1AR. b The results of LDH assay. β1AA significantly increased the apoptosis of H9C2 cells. This effect could also be mimicked by isoprenaline and inhibited by atenolol. c TUNEL positive nuclei obtained from H9C2 cells visualized by fluorescence microscopy. Scale bar, 100 μm. d Group data of apoptosis rate of TUNEL assay (n = 5). E and F. β1AA significantly inreased the mRNA and protein expression of the β1AR (n = 5). The effects of β1AA on β1AR could be inhibited by atenolol. **, P ≤ 0.01. Group data presented by mean ± SEM
β1AA inhibited the PGC-1α related factor expression
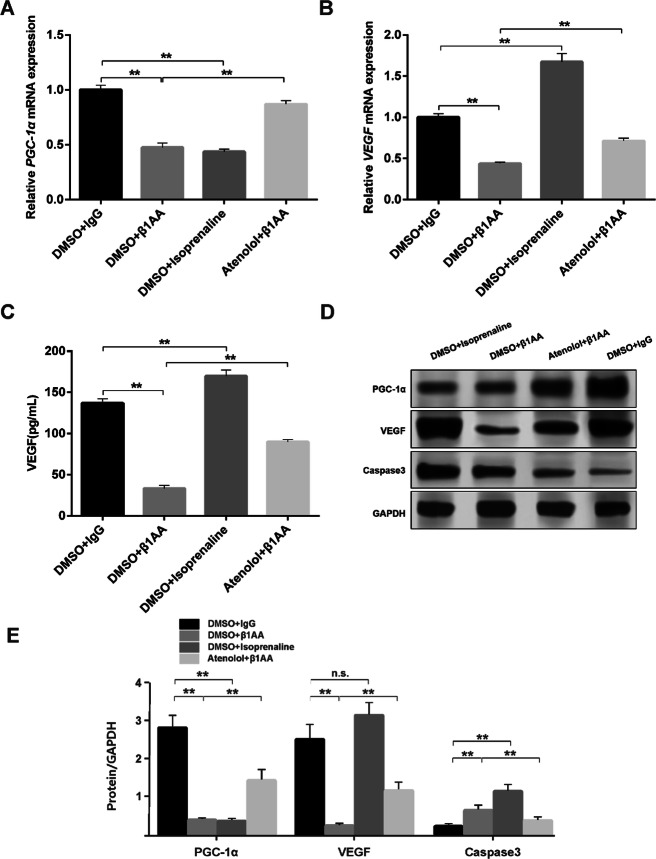
We examined the PGC-1α related factor expression after β1AA treatment. Both β1AA and isoprenaline, inhibited the expression of PGC-1α mRNA and atenolol inhibited the effect of β1AA (Fig. 3a). Furthermore, β1AA inhibited the expression of VEGF mRNA, while isoprenaline promoted its expression. Atenolol also inhibited the effect of β1AA on the expression of VEGF mRNA (Fig. 3b). The results of the ELISA test for secreted VEGF in the supernatant of the medium show that β1AA inhibited VEGF secretion and atenolol inhibited the effect of β1AA (Fig. 3c). Isoprenaline promoted the secretion of VEGF, which was the same as mRNA results (Fig. 3c). In addition, as shown in Fig. 3d and e, the expression of caspase3 protein increased obviously, while the expression of PGC-1α and VEGF protein decreased after β1AA treatment. Atenolol can inhibit these effects. Although isoprenaline inhibited the expression of PGC-1α protein and activated the expression of caspase3 protein like β1AA, whereas increased the expression of VEGF, which is contrary to β1AA. Altogether, these results suggested that β1AA could inhibit the PGC-1α related factor expression.
Fig. 3.
β1AA inhibited the PGC-1α related factor expression. β1AA significantly inhibited the mRNA expression of PGC-1α (a) and VEGF (b). And the effect of β1AA on mRNA expression of PGC-1α could also be mimicked by isoprenaline. However, isoprenaline promoted the mRNA expression of VEGF (n = 5). cELISA test for secreted VEGF in the supernatant of the medium (n = 5). d Raw data of results of WB test. e Relative expression content of PGC-1α, VEGF and caspase3 (n = 5). β1AA increased the protein expression of caspase3, and inhibited the protein expression of PGC-1α and VEGF. The effects of β1AA could be inhibited by atenolol. And the effect of β1AA on protein expression of PGC-1α could also be mimicked by isoprenaline. However, isoprenaline promoted the protein expression of VEGF expression rather than inhibition as β1AA did. **, P ≤ 0.01. n.s., no statistical significance. Group data presented by mean ± SEM
Overexpression of PGC-1α rescued β1AA induced apoptosis of H9C2 cells
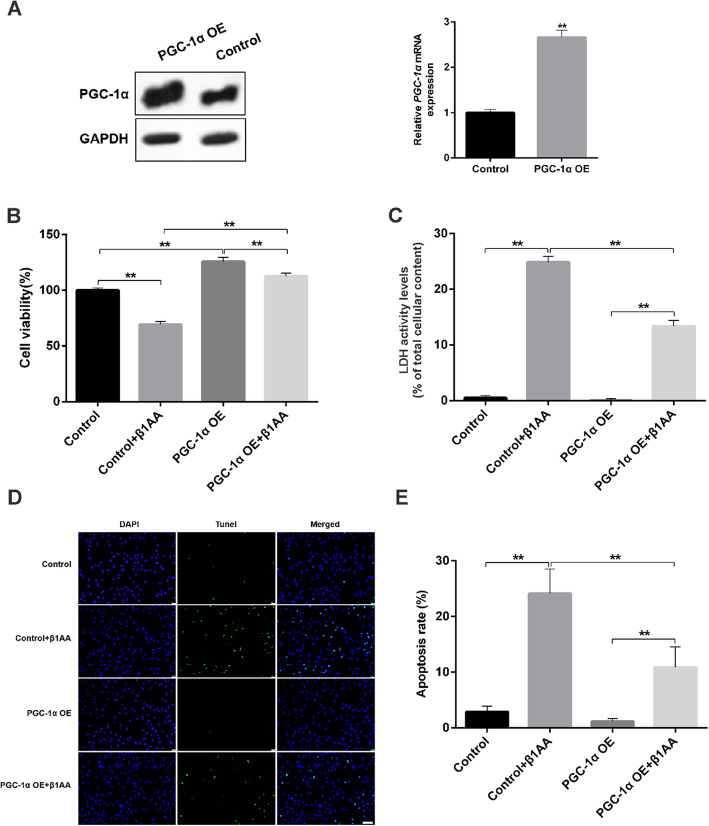
Next, we overexpressed PGC-1α in H9C2 cells. The expression of mRNA and protein of PGC-1α had a significant increase in the overexpressed H9C2 cells (Fig. 4a). It suggested the successful build-up of the cell models. Then we tested the cell proliferation by CCK8 assay and found that β1AA inhibited cell proliferation compared to the control group in the cells transfusing empty plasmids. However, overexpression of PGC-1α significantly rescued the inhibition of β1AA to cell proliferation (Fig. 4b). We obtained similar results in the LDH (Fig. 4c) and Tunel assay (Fig. 4d and e). The apoptosis of H9C2 cells significantly increased after β1AA treatment. But overexpression of PGC-1α significantly decreased the apoptosis rate of the cells and inhibited the role of β1AA in promoting apoptosis.
Fig. 4.
Overexpression of PGC-1α rescued β1AA induced apoptosis of H9C2 cells. a The expression of protein and mRNA of PGC-1α in the PGC-1α overexpression H9C2 cells. b Overexpression of PGC-1α rescued the inhibition of β1AA to cell proliferation in CCK8 assay. c Overexpression of PGC-1α decreased the apoptosis of the cells and inhibited the role of β1AA in promoting apoptosis in LDH assay. d TUNEL positive nuclei obtained from H9C2 cells visualized by fluorescence microscopy. Scale bar, 100 μm. e Group data of apoptosis rate of TUNEL assay. **, P ≤ 0.01. Group data presented by mean ± SEM
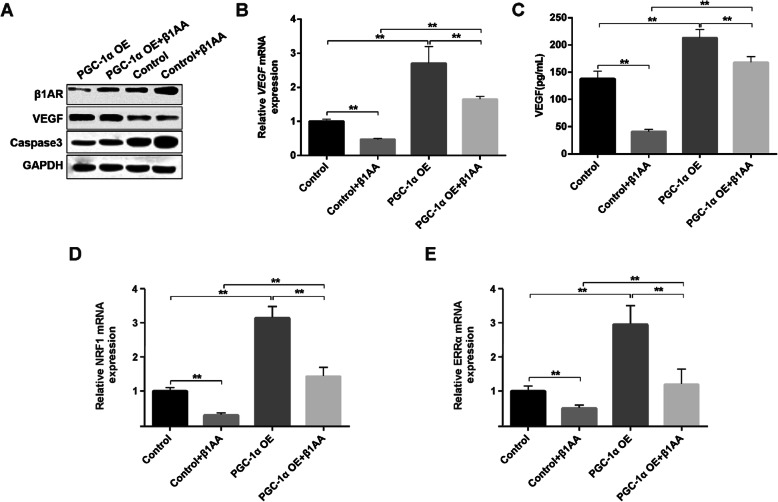
Overexpression of PGC-1α rescued the inhibitory effect of β1AA on VEGF expression
The expression of β1AR and caspase3 protein increased and, the expression of VEGF protein decreased after β1AA treatment in the cells transfusing empty plasmids (Fig. 5a and b). In comparison to cells transfected with empty plasmids, cells with overexpressed PGC-1α had lower expression of β1AR, caspase 3 proteins, and increased expression of VEGF protein, even after β1AA treatment. We also tested the expression of VEGF mRNA in each group. The results showed that the β1AA significantly inhibited the expression of VEGF mRNA, and the overexpression of PGC-1α rescued the inhibitory effect of β1AA on expression of VEGF mRNA (Fig. 5c). We found similar results in ELISA test for secreted VEGF in the supernatant of the medium. β1AA significantly inhibited the VEGF secretion, and PGC-1α overexpression rescued the inhibitory effect of β1AA on secretion of VEGF. Because it has been reported that PGC-1α could regulate mitochondrial biogenesis and function [11, 12]. Finally, we tested the mRNA expression of NRF1 and ERRα, which were associated with mitochondrial biogenesis. Quantitative PCR analyses showed specific down-regulation of NRF1 and ERRα after β1AA treatment and it was rescued by overexpression of PGC-1α. All these results suggest that β1AA inducing myocardial cell apoptosis is by inhibiting the PGC-1α pathway, which leads to disorder of cell mitochondrial metabolism.
Fig. 5.
Overexpression of PGC-1α rescued the inhibitory effect of β1AA on VEGF expression. a Raw data of results of WB test. β1AA increased the protein expression of β1AR and caspase3, and inhibited the protein expression of VEGF. But the overexpression of PGC-1α inhibited the effect of β1AA. b Overexpression of PGC-1α increased the VEGF mRNA expression and rescued the inhibitory effect of β1AA. c ELISA test for secreted VEGF in the supernatant of the medium. Overexpression of PGC-1α also rescued the inhibitory effect of β1AA on the VEGF secretion. d and e overexpression of PGC-1α increased the NRF1 and ERRα mRNA expression and rescued the inhibitory effect of β1AA. **, P ≤ 0.01. Group data presented by mean ± SEM
Discussion
The Heart Failure Association of the European Society of Cardiology Working Group defined PPCM as idiopathic cardiomyopathy presenting with heart failure secondary to left ventricular systolic dysfunction towards the end of pregnancy or in the months following delivery, where no other cause of heart failure is found [4]. PPCM occurs in 1/1000–1/4000 pregnancies, more commonly in women of African ancestry [13]. Perhaps it is found up to 1% of all pregnancies in countries like Haiti and Nigeria [14, 15]. Only 25% of PPCM patients in developing countries survive up to 5 years, with associated infant mortality of 50–75% [16]. Our results showed that β1AA, which were extracted from postpartum Wistar rats that were injected by the antigen peptide segment of the β1 adrenoceptor, could induce apoptosis of cardiac myocytes of H9C2 cell line and the effect of β1AA on H9C2 cells might be caused by inhibition of PGC-1α related pathway.
Although the pathophysiological mechanism is not known, abnormal autoimmunity response to pregnancy and β1AA are associated with high risk for the progression and prevalence of PPCM. Because the serum level of β1AA in patients with DCM is higher than in normal people [17, 18] and later the immunization of animals with a synthetic peptide corresponding to the second extracellular loop of β1AR leads to the production of IgG autoantibodies against this domain [19]. After several months of immunization, the animals showed left ventricular hypertrophy and contractile dysfunction [20]. β-blockers have been used successfully for decades to treat several pathologies, including hypertension, congestive heart failure, and post-MI dysfunction [21]. Moreover, chronic β-blocker treatment could alter baseline leukocyte characteristics that decrease their responsiveness to acute injury, and it means prior β-blockade may act to reduce the severity of innate immune responses [22]. Since β1AA is a weak agonist of β1AR, its accumulation might lead to the opposite effect of the blockers. Finally, as an adrenoceptor ligand, the sirloin protein fully involves in cardiac inflammatory, oxidative, and apoptotic processes [23, 24]. Although in diabetic patients, the protective anti-apoptotic and anti-remodeling effect played by drugs on the cardiovascular system not implies in the regulation of sympathetic tone and β-adrenoreceptors [25, 26], our previous study found that there was a high correlation between β1AA and development of PPCM [3]. This difference may be due to the effects of different diseases on cardiomyocytes. Most recently, experimental progress has strongly suggested a causal role for hormonal insults in PPCM [2]. However, there is no direct evidence for β1AA induced PPCM. Here, we first proved that β1AA, which were extracted from the postpartum rats with autoimmunity, directly induce apoptosis in H9C2 cardiac myocytes through β1AR. It signifies that apoptosis plays a pathophysiological role in heart failure [27]. Thus, these results suggest that β1AA may directly contribute to the pathogenesis of PPCM.
Our data show that β1AA inhibited the PGC-1α related factor expression and VEGF secretion. Moreover, PGC-1α overexpression rescued apoptosis of cardiac myocytes induced by β1AA and the inhibitory effect of β1AA on PGC-1α related factor expression and VEGF secretion. It suggests that the mechanism of β1AA induced apoptosis is through inhibiting the PGC-1α related pathway. PGC-1α plays a critical role in the augmentation of mitochondrial biogenesis, cellular respiration rates, and energy substrate uptake and utilization [10]. Down regulation of the PGC-1α has been described both in patients with nonischemic cardiomyopathy and in animal models of pressure overload and heart failure [28–30]. Furthermore, in the PGC-1α-KO models, PGC-1α deletion resulted in reduced palmitate oxidation and increased glucose oxidation. PGC-1α-KO hearts exhibited impaired inotropic and chronotropic response [31–33]. In addition, in present study, β1AA showed specific down-regulation of genes associated with mitochondrial biogenesis (NRF1 and ERRα). Therefore, the inhibitory effect of β1AA on PGC-1α related factor expression might cause mitochondria dysfunction, later leading to heart failure. On the other hand, under the ischemia condition, PGC-1α expression can induce skeletal muscle cells leading to VEGF secretion to regenerate the blood vessels [34]. Recently, heart failure remains associated with microvascular sparsity [35]. Our data shows β1AA inhibition of VEGF secretion, leading to a decrease in microvascular density, suggesting another reason for β1AA induced PPCM. The diagnosis of PPCM is difficult to make, as it is a diagnosis of exclusion, with a considerable overlap with other conditions. Especially in women who present with acute heart failure at the end of pregnancy or directly postpartum, thorough investigations and intensive follow-up may often lead to alternative diagnoses [4]. Our findings suggest the increase of β1AA together with the decrease of PGC-1α as a diagnostic tool for PPCM. However, more clinical studies are needed to prove this deduction.
β1AA are the weak agonist of β1AR. We also found atenolol, the antagonist of β1AR can inhibit the effect of β1AA, suggesting the function of β1AA, realized through β1AR. However, isoprenaline is the non-selective agonist of β adrenoceptor, it cloud active both β1AR and β2 adrenoceptors (β2AR) [1]. The difference between effects of β1AA and isoprenaline on the expression of β1AR might be caused by mechanism of function, including receptor affinity and dynamic properties of the β-agonist. Activation of β2AR has been reported to promote VEGF secretion [36] and restore mitochondrial function of kidney [37] and podocyte [38] through PGC-1α dependent mitochondrial biogenesis. It seems that the activation of β2AR would lead to increase of VEGF and PGC-1α expression. We thought the different effects of isoprenaline on PGC-1α and VEGF are the result of the integration of its activation of β1AR and β2AR at the same time. However, the effects of activations of different β adrenergic receptor on expression of β adrenergic receptor and PGC-1α related pathway should be further studied.
Conclusions
In conclusion, our present findings establish that β1AA directly induce apoptosis in H9C2 cardiac myocytes through β1AR. The reduced expression of PGC-1α and VEGF, as well as the consequently elevated apoptosis rate of myocytes, may account for the β1AA-induced PPCM. Therefore, our results uncover a previously unknown role of the β1AA pathway in the etiology of PPCM,and provide a novel potential target for the treatment of PPCM.
Acknowledgements
The authors thank the Dr. Lei YU from Nanjing University of Traditional Chinese Medicine, China for editing this manuscript.
Abbreviations
- β1AR
β1 adrenoceptors
- β2AR
β2 adrenoceptors
- β1AA
β1 adrenoceptor antibodies
- DCM
Dilated cardiomyopathy
- PPCM
Peripartum cardiomyopathy
- PGC-1α
Peroxisome proliferator-activated receptor γ coactivator-1α
- VEGF
Vascular endothelial growth factor
Authors’ contributions
LS and JL performed the cell experiments; YZ extracted the extractive β1AA from animals; LS and MC helped perform statistical analysis of results and wrote related parts of the manuscript. JML designed the work and wrote the final manuscript. All authors gave input to the manuscript.
Funding
This work was supported by the National Science Foundation of China to J-M. L (81471477). The funding were used for the reagent, animals, enrollment, academic communication, labor costs and so on.
Availability of data and materials
The dataset used and/ or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
All animal experiments were approved by the Institutional Animal Care and Use Committee (IACUC) of Nanjing University, Nanjing, China, and was conducted in accordance with the Guide for the Care and Use of Laboratory Animals from the National Institutes of Health (Bethesda, MD, USA).
Consent for publication
Not applicable.
Competing interests
The authors have declared that no Competing interests exist.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Linying Shi, Email: yang96353@sohu.com.
Jia Liu, Email: 15501251696@126.com.
Yuan Zhang, Email: my55bbs1@126.com.
Mulei Chen, Email: cml1968@sina.com.
Jiamei Liu, Email: liujiamei2006@yeah.net.
References
- 1.Najafi A, Sequeira V, Kuster DW, van der Velden J. β-adrenergic receptor signalling and its functional consequences in the diseased heart. Eur J Clin Invest. 2016;46:362–374. doi: 10.1111/eci.12598. [DOI] [PubMed] [Google Scholar]
- 2.Arany Z, Elkayam U. Peripartum cardiomyopathy. Circulation. 2016;133:1397–1409. doi: 10.1161/CIRCULATIONAHA.115.020491. [DOI] [PubMed] [Google Scholar]
- 3.Liu J, Wang Y, Chen M, Zhao W, Wang X, Wang H, Zhang Z, Zhang J, Xu L, Chen J, Yang X, Zhang L. The correlation between peripartum cardiomyopathy and autoantibodies against cardiovascular receptors. PLoS One. 2014;9:e86770. doi: 10.1371/journal.pone.0086770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baris L, Cornette J, Johnson MR, Sliwa K, Roos-Hesselink JW. Peripartum cardiomyopathy: disease or syndrome? Heart. 2019;105:357–362. doi: 10.1136/heartjnl-2019-315060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Finck BN, Kelly DP. Peroxisome proliferator-activated receptor gamma coactivator-1 (PGC-1) regulatory cascade in cardiac physiology and disease. Circulation. 2007;115:2540–2548. doi: 10.1161/CIRCULATIONAHA.107.670588. [DOI] [PubMed] [Google Scholar]
- 6.Finck BN, Kelly DP. PGC-1 coactivators: inducible regulators of energy metabolism in health and disease. J Clin Invest. 2006;116:615–622. doi: 10.1172/JCI27794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Murphy E, Ardehali H, Balaban RS, DiLisa F, Dorn GW, 2nd, Kitsis RN, Otsu K, Ping P, Rizzuto R, Sack MN, Wallace D, Youle RJ. American Heart Association Council on Basic Cardiovascular Sciences, Council on Clinical Cardiology, and Council on Functional Genomics and Translational Biology. Mitochondrial Function, Biology, and Role in Disease: A Scientific Statement From the American Heart Association. Circ Res. 2016;118:1960–1991. doi: 10.1161/RES.0000000000000104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Staudt Y, Mobini R, Fu M, Felix SB, Kühn JP, Staudt A. Beta1-adrenoceptor antibodies induce apoptosis in adult isolated cardiomyocytes. Eur J Pharmacol. 2003;466:1–6. doi: 10.1016/S0014-2999(03)01431-6. [DOI] [PubMed] [Google Scholar]
- 9.Huss JM, Kelly DP. Mitochondrial energy metabolism in heart failure: a question of balance. J Clin Invest. 2005;115:547–555. doi: 10.1172/JCI24405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sen N, Satija YK, Das S. PGC-1α, a key modulator of p53, promotes cell survival upon metabolic stress. Mol Cell. 2011;44:621–634. doi: 10.1016/j.molcel.2011.08.044. [DOI] [PubMed] [Google Scholar]
- 11.Khodambashi Emami N, Golian A, Danesh Mesgaran M, Anthony NB, Rhoads DD. Mitochondrial biogenesis and PGC-1α gene expression in male broilers from ascites-susceptible and -resistant lines. J Anim Physiol Anim Nutr (Berl) 2018;102:e482–e485. doi: 10.1111/jpn.12706. [DOI] [PubMed] [Google Scholar]
- 12.Wu Z, Puigserver P, Andersson U, Zhang C, Adelmant G, Mootha V, Troy A, Cinti S, Lowell B, Scarpulla RC, Spiegelman BM. Mechanisms controlling mitochondrial biogenesis and respiration through the thermogenic coactivator PGC-1. Cell. 1999;98:115–124. doi: 10.1016/S0092-8674(00)80611-X. [DOI] [PubMed] [Google Scholar]
- 13.Kolte D, Khera S, Aronow WS, Palaniswamy C, Mujib M, Ahn C, Jain D, Gass A, Ahmed A, Panza JA, Fonarow GC. Temporal trends in incidence and outcomes of Peripartum cardiomyopathy in the United States: a Nationwide population-based study. J Am Heart Assoc. 2014;3:e001056. doi: 10.1161/JAHA.114.001056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fett JD, Christie LG, Carraway RD, Murphy JG. Five-year prospective study of the incidence and prognosis of peripartum cardiomyopathy at a single institution. Mayo Clin Proc. 2005;80:1602–1606. doi: 10.4065/80.12.1602. [DOI] [PubMed] [Google Scholar]
- 15.Isezuo SA, Abubakar SA. Epidemiologic profile of peripartum cardiomyopathy in a tertiary care hospital. Ethn Dis. 2007;17:228–233. [PubMed] [Google Scholar]
- 16.Fett JD, Murphy JG. Infant survival in Haiti after maternal death from peripartum cardiomyopathy. Int J Gynaecol Obstet. 2006;94:135–136. doi: 10.1016/j.ijgo.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 17.Limas CJ, Goldenberg IF, Limas C. Autoantibodies against betaadrenoceptors in human idiopathic dilated cardiomyopathy. Circ Res. 1989;1:97–103. doi: 10.1161/01.RES.64.1.97. [DOI] [PubMed] [Google Scholar]
- 18.Magnusson Y, Marullo S, Hoyer S, Waagstein F, Andersson B, Vahlne A, Guillet JG, Strosberg AD, Hjalmarson A, Hoebeke J. Mapping of a functional autoimmune epitope on the beta 1-adrenergic receptor in patients with idiopathic dilated cardiomyopathy. J Clin Invest. 1990;5:1658–1663. doi: 10.1172/JCI114888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Iwata M, Yoshikawa T, Baba A, Anzai T, Nakamura I, Wainai Y, Takahashi T, Ogawa S. Autoimmunity against the second extracellular loop of beta(1)-adrenergic receptors induces beta-adrenergic receptor desensitization and myocardial hypertrophy in vivo. Circ Res. 2001;6:578–586. doi: 10.1161/01.RES.88.6.578. [DOI] [PubMed] [Google Scholar]
- 20.Liu HR, Zhao RR, Jiao XY, Wang YY, Fu M. Relationship of myocardial remodeling to the genesis of serum autoantibodies to cardiac beta(1)-adrenoceptors and muscarinic type 2 acetylcholine receptors in rats. J Am Coll Cardiol. 2002;11:1866–73. [DOI] [PubMed]
- 21.Bristow MR. Treatment of chronic heart failure with β-adrenergic receptor antagonists: a convergence of receptor pharmacology and clinical cardiology. Circ Res. 2011;109(10):1176–1194. doi: 10.1161/CIRCRESAHA.111.245092. [DOI] [PubMed] [Google Scholar]
- 22.Grisanti LA, de Lucia C, Thomas TP, Stark A, Strony JT, Myers VD, Beretta R, Yu D, Sardu C, Marfella R, Gao E, Houser SR, Koch WJ, Hamad EA, Tilley DG. Prior β-blocker treatment decreases leukocyte responsiveness to injury. JCI Insight. 2019;5:e99485. [DOI] [PMC free article] [PubMed]
- 23.Sardu C, Pieretti G, D'Onofrio N, Ciccarelli F, Paolisso P, Passavanti MB, Marfella R, Cioffi M, Mone P, Dalise AM, Ferraraccio F, Panarese I, Gambardella A, Passariello N, Rizzo MR, Balestrieri ML, Nicoletti G, Barbieri M. Inflammatory cytokines and SIRT1 levels in subcutaneous abdominal fat: relationship with cardiac performance in overweight pre-diabetics patients. Front Physiol. 2018;9:1030. doi: 10.3389/fphys.2018.01030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.D'Onofrio N, Pieretti G, Ciccarelli F, Gambardella A, Passariello N, Rizzo MR, Barbieri M, Marfella R, Nicoletti G, Balestrieri ML, Sardu C. Abdominal Fat SIRT6 Expression and Its Relationship with Inflammatory and Metabolic Pathways in Pre-Diabetic Overweight Patients. Int J Mol Sci. 2019;20. [DOI] [PMC free article] [PubMed]
- 25.Marfella R, Sardu C, Balestrieri ML, Siniscalchi M, Minicucci F, Signoriello G, Calabrò P, Mauro C, Pieretti G, Coppola A, Nicoletti G, Rizzo MR, Paolisso G, Barbieri M. Effects of incretin treatment on cardiovascular outcomes in diabetic STEMI-patients with culprit obstructive and multivessel non obstructive-coronary-stenosis. Diabetol Metab Syndr. 2018;3(10):1. doi: 10.1186/s13098-017-0304-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marfella R, Sardu C, Calabrò P, Siniscalchi M, Minicucci F, Signoriello G, Balestrieri ML, Mauro C, Rizzo MR, Paolisso G, Barbieri M. Non-ST-elevation myocardial infarction outcomes in patients with type 2 diabetes with non-obstructive coronary artery stenosis: effects of incretin treatment. Diabetes Obes Metab. 2018;20:723–729. doi: 10.1111/dom.13122. [DOI] [PubMed] [Google Scholar]
- 27.Preedy MEJ, Baliga RS, Hobbs AJ. Multiplicity of nitric oxide and natriuretic peptide Signalling in heart failure. J Cardiovasc Pharmacol. 2019. 10.1097/FJC.0000000000000724 [Epub ahead of print]. [DOI] [PubMed]
- 28.Davila-Roman VG, Vedala G, Herrero P, de las Fuentes L, Rogers JG, Kelly DP, Gropler RJ. Altered myocardial fatty acid and glucose metabolism in idiopathic dilated cardiomyopathy. J Am Coll Cardiol. 2002;40:271–277. doi: 10.1016/S0735-1097(02)01967-8. [DOI] [PubMed] [Google Scholar]
- 29.Razeghi P, Young ME, Alcorn JL, Moravec CS, Frazier OH, Taegtmeyer H. Metabolic gene expression in fetal and failing human heart. Circulation. 2001;104:2923–2931. doi: 10.1161/hc4901.100526. [DOI] [PubMed] [Google Scholar]
- 30.Sack MN, Rader TA, Park S, Bastin J, McCune SA, Kelly DP. Fatty acid oxidation enzyme gene expression is downregulated in the failing heart. Circulation. 1996;94:2837–2842. doi: 10.1161/01.CIR.94.11.2837. [DOI] [PubMed] [Google Scholar]
- 31.Arany Z, He H, Lin J, Hoyer K, Handschin C, Toka O, Ahmad F, Matsui T, Chin S, Wu PH, et al. Transcriptional coactivator PGC-1 alpha controls the energy state and contractile function of cardiac muscle. Cell Metab. 2005;1:259–271. doi: 10.1016/j.cmet.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 32.Lehman JJ, Boudina S, Banke NH, Sambandam N, Han X, Young DM, Leone TC, Gross RW, Lewandowski ED, Abel ED, et al. The transcriptional coactivator PGC-1alpha is essential for maximal and efficient cardiac mitochondrial fatty acid oxidation and lipid homeostasis. Am J Physiol Heart Circ Physiol. 2008;295:H185–H196. doi: 10.1152/ajpheart.00081.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Leone TC, Lehman JJ, Finck BN, Schaeffer PJ, Wende AR, Boudina S, Courtois M, Wozniak DF, Sambandam N, Bernal-Mizrachi C, et al. PGC-1alpha deficiency causes multi-system energy metabolic derangements: muscle dysfunction, abnormal weight control and hepatic steatosis. PLoS Biol. 2005;3:e101. doi: 10.1371/journal.pbio.0030101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Arany Z, Foo SY, Ma Y, Ruas JL, Bommi-Reddy A, Girnun G, Cooper M, Laznik D, Chinsomboon J, Rangwala SM, et al. HIF-independent regulation of VEGF and angiogenesis by the transcriptional coactivator PGC-1alpha. Nature. 2008;451:1008–1012. doi: 10.1038/nature06613. [DOI] [PubMed] [Google Scholar]
- 35.Mohammed SF, Hussain S, Mirzoyev SA, Edwards WD, Maleszewski JJ, Redfield MM. Coronary microvascular rarefaction and myocardial fibrosis in heart failure with preserved ejection fraction. Circulation. 2015;131:550–559. doi: 10.1161/CIRCULATIONAHA.114.009625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Garg J, Feng YX, Jansen SR, Friedrich J, Lezoualc'h F, Schmidt M, Wieland T. Catecholamines facilitate VEGF-dependent angiogenesis via β2-adrenoceptor-induced Epac1 and PKA activation. Oncotarget. 2017;8:44732–44748. doi: 10.18632/oncotarget.17267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Arif E, Nihalani D. Beta2-adrenergic receptor in kidney biology: a current prospective. Nephrology (Carlton) 2019;24:497–503. doi: 10.1111/nep.13584. [DOI] [PubMed] [Google Scholar]
- 38.Arif E, Solanki AK, Srivastava P, Rahman B, Fitzgibbon WR, Deng P, Budisavljevic MN, Baicu CF, Zile MR, Megyesi J, Janech MG, Kwon SH, Collier J, Schnellmann RG, Nihalani D. Mitochondrial biogenesis induced by the β2-adrenergic receptor agonist formoterol accelerates podocyte recovery from glomerular injury. Kidney Int. 2019;96:656–673. doi: 10.1016/j.kint.2019.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The dataset used and/ or analyzed during the current study are available from the corresponding author on reasonable request.