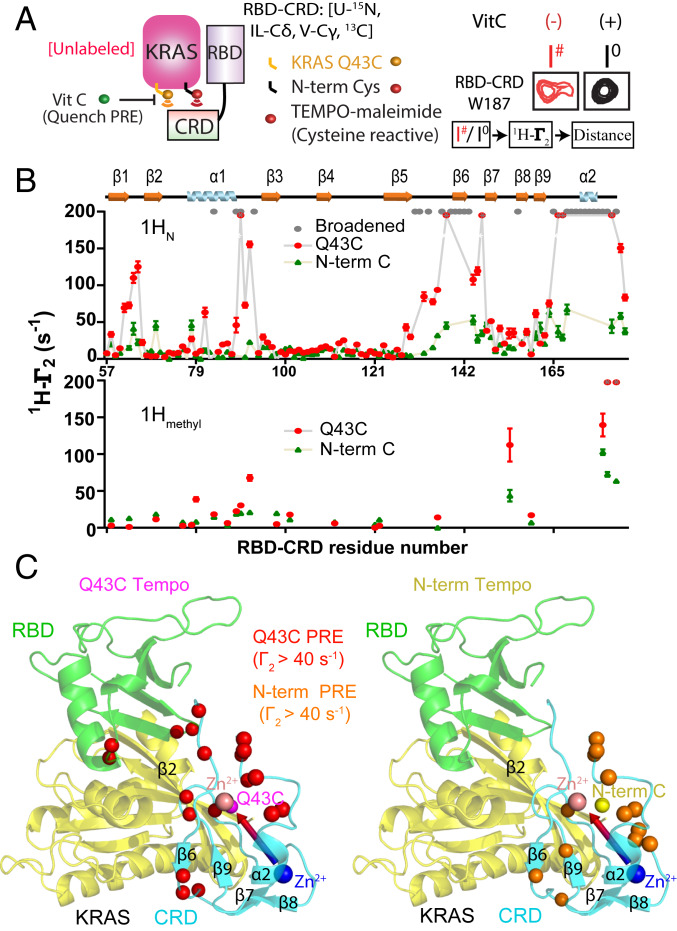
Fig. 2.
Site-directed spin-labeling and PRE reveal an RBD-dependent interaction between KRAS and the CRD domain of CRAF. (A) Schematic diagram of spin-labeled KRAS and the resulting PRE on RBD–CRD. TEMPO-maleimide was covalently linked to one of two cysteine residues introduced to KRAS near switch I (Q43C) or at the N terminus (Cys0). Interaction with RBD–CRD caused PRE-induced broadening of peaks from proximal residues (e.g., W187, Right). I#/Io is the ratio of peak heights before and after quenching the TEMPO spin label with Vitamin C (Vit C). (B) Estimated 1HN-Γ2 rates of KRAS Q43C-TEMPO (red) or N-term-TEMPO (green) on 15N, 13C Ile-Cδ, Val-Cγ, Leu-Cδ, Met-Cε-RBD–CRD amide, and methyl resonances. Residues broadened beyond detection after adding KRAS (even in the absence of TEMPO) are colored gray. Residues broadened beyond detection in the presence of TEMPO are colored in red with a gray asterisk, and their 1HN-Γ2 rates are assigned to a fixed value of 200. (C) Structural model of KRAS in complex with RBD–CRD. The positions of Q43C and the N-term Cys (Cys0) in KRAS are shown in pink and yellow, respectively. Residues exhibiting strong PRE from TEMPO tags attached to Cys43 or N-term are colored red or orange, respectively.