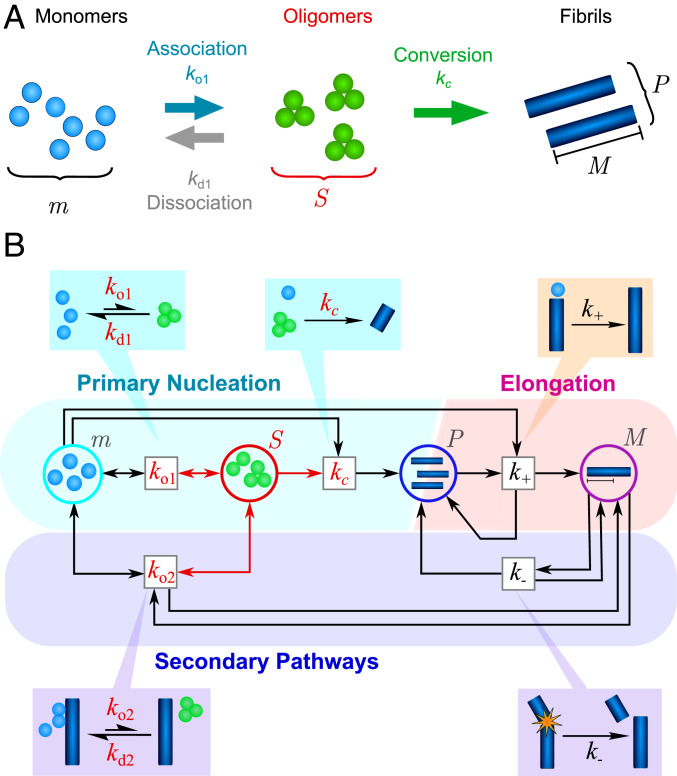
Fig. 1.
(A) The different possible reactions that can produce and deplete nonfibrillar amyloid oligomers. Oligomers (concentration ) may be generated by free association of monomeric protein (concentration ) with rate constant . Once formed, they may dissociate back to monomers (rate constant ) or undergo a structural conversion process to generate fibrillar species (concentration ) with rate constant . Reverse conversion of fibrils to oligomers is neglected as it is found experimentally that fibrils are far more thermodynamically stable than nonfibrillar oligomers. (B) The coarse-grained reaction network describing oligomer-mediated fibril formation, represented in Petri net form (34), with reactions represented by boxes and chemical species of interest as circles. Oligomers, and reactions involving them, are highlighted in red. Both oligomer formation through monomer association and oligomer dissociation may in some cases be catalyzed by the surfaces of existing fibrils (fibril mass concentration ) with rate constants and , respectively. Once formed, fibrillar species undergo rapid elongation by monomer addition (rate constant ), increasing the fibril mass concentration. They may also fragment to generate new fibrils (rate constant ), increasing .