Figure 6.
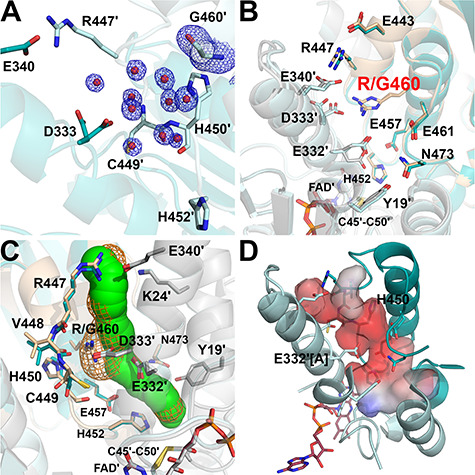
Structure of the R460G-hE3 variant (PDB ID: 6I4R). (A) (2mFo-DFc) composite omit map (1.5σ) confirms the R460G substitution [Arg side chain was replaced with H2O (red spheres)] in R460G-hE3. The two chains are colored with two shades of teal. (B) H+/H2O channel and relevant polar side chains are shown (with the Pro16-Gly29 helix partially omitted, see Fig. 3C and D) in the superimposed R460G-hE3 (dark/light teal; FAD′: raspberry) and hE3 (beige/grey; FAD′: beige) structures. Glu332 was modeled with a single conformer in R460G-hE3. (C) H+/H2O channels computed by Caver are shown in the superimposed hE3 (green) and R460G-hE3 (orange mesh) structures; only dominant conformers were considered for calculation. (D) Surface polarity analysis of the LA-binding and H+/H2O channels in R460G-hE3 is presented using an arbitrary scale (red: negative, blue: positive); orientation and secondary structural elements are as for hE3 in Fig. 3C and D.
