Abstract
Background:
DNA methylation is an epigenetic modification that has the ability to alter gene expression and function. These epigenetic changes have been associated with the development of cancer. Previous research has found that DNA methylation patterns can predict disease prognosis for patients with Acute Promyelocytic Leukemia (APL). The role of DNMT1 and CDH1 in regulating the extension of cells are studied in this study.
Methods:
DNA was extracted from peripheral blood samples of APL patients and treated with bisulfite. DNMT1 and CDH1 gene promoter methylation was subsequently analyzed using methylation-specific PCR (MSP). Real-time PCR was used to measure the expression level of DNMT1 and CDH1 genes.
Results:
Partial methylation of the CDH1 gene promoter was detected in 20% of APL patients and an unmethylated status was detected in 80% of patient samples. Additionally, an unmethylated status in the DNMT1 gene promoter was detected in 100% of APL patient samples.
Conclusion:
Our study found the CDH1 gene promoter to be unmethylated in almost all APL patients, while the DNMT1 promoter was unmethylated in all APL patients. Furthermore, we observed an increase in both CDH1 and DNMT1 gene expression in APL patients compared to healthy controls. These findings suggest that DNMT1 may not have a specific role in inhibiting CDH1 gene expression in APL. Applying higher resolution techniques would help to better uncover the DNA methylation patterns in patients with APL. Further research is required to determine the role of DNA methylation and CDH1 and DNMT1 gene expression in APL.
Key Words: Acute Promyelocytic Leukemia, CDH1, DNMT, Promoter Methylation
Introduction
Acute myeloid leukemia (AML) is a hematopoietic malignancy in which incompletely differentiated hematopoietic progenitor cells accumulate in the bone marrow and blood, interfering with normal hematopoiesis. Research has shown that the accumulation of mutations and epigenetic modifications are key features in the AML genome. Both of these alterations often occur prior to the development of leukemia and persist in residual disease following therapeutic intervention. Thus, targeting the AML epigenome may help cure the residual disease and prevent cancer recurrence (1). The treatment interventions for AML have remained relatively unchanged over the past few decades, however there have been some improvements in the survival outcomes for younger patients (2). In spite of these improvements, AML treatment interventions are unsuccessful for about 60% of young patients (3). In patients over the age of 60, in which there is an increased frequency of AML, the survival outcomes are even worse. The 5 year survival rate for this population is less than 5% (4). Many factors are responsible for the lack of progression in AML treatment success rates. First, the therapeutic approaches that have been successful in younger patients are highly aggressive. Due to the aggressive nature of these treatments, elderly patients who have poor health and additional comorbidities are unable to tolerate the treatment. In standard therapies, both healthy and leukemic cells are targeted (5). This indiscriminate targeting results in severe treatment-related toxicities. Second, treatment strategies and risk stratification are determined by the different morphologic and cytogenetic features of the patient’s AML. However, the current therapies available do not account for the molecular heterogeneity that is inherent to AML. Finally, treatments that target the leukemic bulk may leave behind residual leukemia stem cells (LSCs). These remaining cells can act as a reservoir for precancerous or cancerous clones that may eventually proliferate and result in tumor regrowth. Unfortunately, most patients who enter remission after treatment relapse within the first few years. The occurrence of relapse significantly decreases their chances of survival. Therefore, the lack of treatment options for the majority of AML patients necessitates the development of safer and more effective therapies (6, 7).
Acute Promyelocytic Leukemia (APL) is a unique subtype of AML that has been classified as M3 by the French-American-British (FAB) cooperative group (8). The frequency of APL among AML cases varies from 2% in Switzerland to over 50% in Nicaragua (9). This subtype of AML is characterized by the reciprocal translocation between chromosome 15 and 17 [t(15;17)]; Promyelocytic leukemia gene (PML)- retinoic acid receptor gene alpha (RARA) fusion] (10). This fusion protein negatively impacts the differentiation and maturation of myeloid progenitor cells such that they remain in the promyelocytic stage (11, 12).
It has been shown that in addition to the accumulation of genetic mutations in oncogenes and tumor suppressor genes (TSGs), epigenetic alterations can lead to carcinogenesis (13, 14). In AML there are fewer mutations than other cancers (13). This characteristic suggests that epigenetic changes, a mechanism of regulating gene expression in which the nucleotide sequence is not altered (15-17), play a significant role in determining the biological behavior of the disease. The most prevalent epigenetic mechanism is DNA methylation occurring at cytosine in CpG dinucleotides. In the regulatory region of many genes, including TSGs, there are CpG-rich regions. Generally, these CpG islands are unmethylated, however some repetitive genomic sequences and introns are hypermethylated (18, 19). DNMTs catalyze the addition of a methyl group at the carbon 5 position of the cytosine ring (19). Different DNMT isoforms, especially DNMT1 has been shown to have an essential role in the regulation of hematopoietic stem cell (HSC) growth, differentiation and survival. Therefore, it is reasonable to hypothesize that abnormal methylation of DNMT leads to the development leukemogenesis (20, 21). Additionally, there is evidence for an up-regulation of DNMT1 activity in cancer (22).
The E-cadherin cell-cell adhesion glycoprotein encoded by the CDH1 gene (also known as Ecad) contains five extracellular cadherin repeats, a transmembrane region, and a highly conserved cytoplasmic tail, and its function is calcium dependent. The CDH1 gene is a TSG that holds a critical role in maintaining cell adhesion and adherent junctions in normal tissues. CDH1 expression is often downregulated or absent in various epithelial tumors. The loss of E-cadherin intercellular junctions contributes to various aspects of cancer progression, including the evolution of drug resistance, angiogenesis, tissue invasion, and metastasis. Due to the dysregulation of DNA methylation and myeloid leukemia, demethylating therapies may be an effective approach for cancer treatment. Additionally, CDH1 is one of the most hypermethylated genes in human cancer (23).
Previous research has explored the relationship between DNMT1 expression and promoter methylation among different TSGs. Given the role of DNMT1 in regulating gene expression and cancer pathogenesis and the role of CDH1 in regulating cell proliferation, we therefore examined the expression and methylation pattern of these genes in patients with APL. This is a critical step in the development of new therapeutics for the treatment of this life-threatening disease.
Materials and methods
Sampling
A total of 20 participants were included in this study. Among them, 15, 8 men and 7 women, were diagnosed with APL with a mean age of 37. The control group included 5 healthy participants with same mean of age. All APL patients were recently diagnosed and had not received any treatment. Consent was obtained from all study participants. Five ml peripheral blood samples were drawn and transferred to the laboratory. The complete blood count (CBC) results of each participant are illustrated in Table 1. This research study was approved by Qazvin University of Medical Sciences.
Table 1.
CBC information of patients.
| Patient | WBC (x103/µl) | Platelet (x103/µl) | Hemoglobin (g/dl) |
|---|---|---|---|
| 1 | 1.5 | 20 | 6 |
| 2 | 2 | 60 | 10 |
| 3 | 1 | 30 | 8 |
| 4 | 2.5 | 60 | 9 |
| 5 | 30 | 70 | 7.5 |
| 6 | 6 | 30 | 10 |
| 7 | 4.6 | 45 | 9 |
| 8 | 0.9 | 20 | 6 |
| 9 | 0.5 | 10 | 7 |
| 10 | 2 | 32 | 7.5 |
| 11 | 1 | 45 | 4.5 |
| 12 | 35 | 9 | 7.6 |
| 13 | 1.8 | 18 | 9.2 |
| 14 | 2.6 | 1 | 5.9 |
| 15 | 0.8 | 13 | 6.3 |
White blood cell isolation and DNA extraction
White blood cells (WBCs) were isolated from the peripheral blood samples via Ficoll-Paque-hypaque method. DNA was extracted using the GENE ALL kit (Cat. No. 105-101) according to the manufacturer’s instructions. The purity of the extracted DNA was evaluated by UV spectrophotometry (NanoDrop) and measuring the ratio of 260/280 and the concentration was determined by measuring the ratio of 260/280. Extracted DNA was then treated with bisulfit by using the EpiTect® Fast Bisulfite Kit (Qiagen.Cat.No.59844) according to the manufacturer’s protocol. For each sample, 40 µl DNA was treated. During the bisulfit treatment, non-methylated cytosine deaminates and converts to uracil while methylated cytosine remains unchanged, leading to distinguishing between the methylation status of targeted sequence after performing methylation specific PCR (MSP).
Total RNA extraction and cDNA synthesis
Total RNA was extracted from the isolated WBCs using RiboEx™ reagent (GENEALL, Cat. No. 315-150). From the purified RNA, 6μl was used for quality control purposes. The remaining RNA was stored at -70 °C or used for cDNA synthesis. The Thermo Scientific kit (Lot: 00456696) was used for cDNA synthesis. To measure the quality and concentration of RNA, Nano drop was used, and light absorption was examined at the ratio of 280/260 nm. The quality of cDNA was controlled by doing PCR with GAPDH primers and electrophoresis on 1.5% agarose gel.
Methylation specific PCR (MSP)
In order to examine the methylation pattern of the DNMT1 and CDH1 gene promoter regions, the MSP method was used. For performing MSP, 4 pairs of primers were applied which were specified for the methylated and un-methylated targeted sequences. Primer sequences are shown in Table 2. All of the MSP primers were designed using the methpimer design tool (http://www.urogene.org/methprimer/). To ensure the specificity of the primers the "by search" tool was applied. MSP was performed at a final volume of 20 µl, the final mixture included 10 µl MSP master mix, 7 µl RNase-free water, 1 µl forward primer, 1 µl reverse primer and 1 µl of treated DNA. Amplification was carried out in a thermocycler under the following conditions: initial activation for 600s at 95 °C, 33 cycles for 15s of denaturation at 94 °C, 30s of annealing at 50 °C for DNMT1 and at 57 °C for CDH1, and 30s extension at 72 °C were repeated with 600s final extension at 72 °C. For controlling and optimizing the MSP reactions, the EpiTect DNA control kit (Qiagen. Cat. No. 59695) was used.
Table 2.
Profile of primers used in MSP.
| Gene | Size (bp) | Cycles | Temp °C | Form | 5’ to 3’ Sequences | |
|---|---|---|---|---|---|---|
| CDH1 | 205 | 33 | 62.2 | Methylated | F | GGTGAATTTTTAGTTAATTAGCGGTAC |
| 59.4 | R | GGTGAATTTTTAGTTAATTAGCGGTAC | ||||
| 212 | 33 | 63.9 | Un-methylated | F | GGTGAATTTTTAGTTAATTAGCGGTAC | |
| 60 | R | ACCCATAACTAACCAAAAACACCA | ||||
| DNMT1 | 197 | 33 | 59.3 | Methylated | F | TTAGTAAATCGTGGAGTTTGGAC |
| 54.3 | R | AACGATAAACGAAAACGACG | ||||
| 199 | 33 | 53.5 | Un-methylated | F | AGTAAATTGTGGAGTTTGGAT | |
| 56.1 | R | AAAAAACAATAAACAAAAACAACATCT | ||||
Real-Time PCR
In order to investigate whether the promoter methylation patterns of the CDH1 and DNMT1 genes correlated with their expression, Real-time PCR was performed. DNMT1 and CDH1 gene expression was detected in both APL patients and the control group. ABI7500 was used for this experiment. For designing primers, the primer3Plus design tool was used and the analysis of primers was performed using NCBI Blast. Primer sequences are shown in Table 3. Melt curve analysis was performed for both genes at the end of each run. Due to their efficiency, the difference was less than 3%, therefore analysis of gene expression was done using the 2-ΔΔCt method showing the expression changes. was 25 μl including: 12.5 µl SYBER GREEN, 8.5 µl ddH2o, 2 µl forward and reverse primers and 2 µl cDNA. GAPDH was selected as internal control gene. To ensure there was no contamination, a negative control of NTC was used in each run.
Table 3.
Profile of primers used in Real time PCR
| Gene | Size (bp) | Temp °C | Form | 5’ to 3’ sequences |
|---|---|---|---|---|
| CDH1 | 92 | 58.4 | F | GGGTTAAGCACAACAGCAAC |
| 60.5 | R | ACCTGACCCTTGTACGTGGT | ||
| DNMT1 | 204 | 62.5 | F | GTGGGGGACTGTGTCTCTGT |
| 58.4 | R | TGAAAGCTGCATGTCCTCAC |
Specificity of experiment was investigated by melt curve and electrophoresis. Real time PCR was performed, the final volume of the mixture
Statistical analysis
For all calculations, Statistical Package for Social Sciences (SPSS) software version 22 was used. The Mann-Whitney U test was used to compare CDH1 and DNMT1 gene expression levels and the CBC of patients in the partial-methylated and unmethylated groups. The correlation between CBC and CDH1 and DNMT1 gene expression was assessed using the spearman correlation coefficient. A p-value of less than 0.05 was considered statistically significant.
Results
DNMT1 and CDH1 promoter methylation status
The MSP data showed that the DNMT1 gene methylation status remained stable among the different APL patients regardless of age or gender. Analysis of band intensities revealed that the methylation pattern of the DNMT1 gene promoter followed an un-methylated status in both APL patients and healthy controls. This was interpreted by observing a distinct band for the un-methylated form and an invisible band for the methylated form, suggesting an un-methylated status of the CpG island of the selected region. It was also depicted that 3 of the APL samples (p2, p11 and p12) showed partial methylation in the CDH1 gene promoter due to having a sharp band with un-methylated primers and weak band with methylated primers. The rest of the APL and control samples had an un-methylated status in the promoter region of the CDH1 gene. To validate these results, controls for methylated and un-methylated sequences were used. These findings are summarized in Figure 1.
Figure 1.
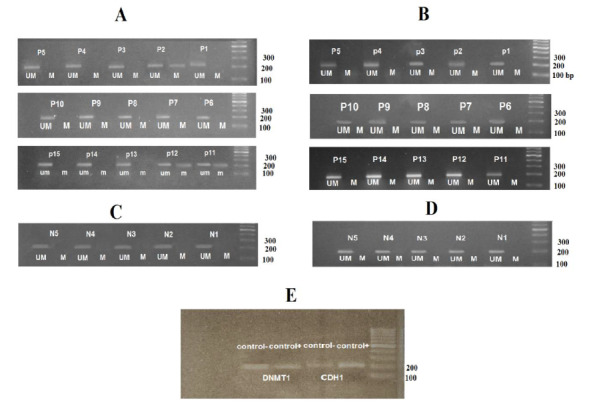
MSP results. A) indicates the methylation status of CDH1 gene in APL patients. B) depicts the methylation status of DNMT1 gene in APL patients. C) MSP results of CDH1 gene in normal samples. D) MSP results of DNMT1 gene in normal samples. E) shows the methylated controls (control+) and un-methylated controls (control-). * M and UM indicate the reactions in which the primers assigned for methylated and un-methylated sequences were used, respectively.
DNMT1 and CDH1 gene expression in APL
The DNMT1 and CDH1 gene expression levels were measured using Real time PCR normalized to the GAPDH expression level. Our findings showed that the levels of both DNTM1 and CDH1 genes were up-regulated compared to controls. The fold changes of both genes are shown in Figure 2. Using the Man Whitney U test, it was concluded that there was no correlation between CDH1 and DNMT1 gene expression and promoter methylation status (p= 0.8 and p= 0.2, respectively). No differences were found between CDH1 and DNMT1 gene expression among male and female APL patients (p= 0.95 and 0.12, respectively). Using spearman correlation coefficient, there was no correlation between CBC results (WBC, platelet and HB) and CDH1 or DNMT1 expression (p> 0.05). Moreover, no correlation was observed between CBC results of APL patients and of the promoter methylation status of the CDH1 or DNMT1 genes.
Figure 2.
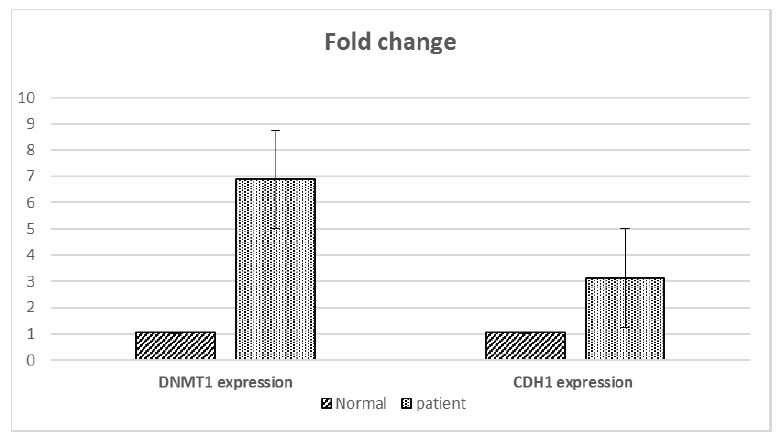
Fold change of CDH1 and DNMT1 genes in patients and controls.
Discussion
Aberrant epigenetic modifications have been associated with the development of cancer. Changes in DNA methylation patterns is one of the most common epigenetic abnormalities associated with cancer. Approximately 80% of CpG sites within the human genome have methylation changes and 70% of sites are methylated at specific times, indicating that the methylation patterns of DNA is modified as a normal means of gene regulation (24).
Unlike genetic mutations, epigenetic modifications are reservable. As a result, targeting cancer-related epigenetic changes is an attractive approach in the development of effective cancer treatments (25-27). Research has shown that nucleoside analogues such as 5-azacyti¬dine and 5-aza-2-deoxycytidine (decitabine) can reactivate hypermethylated gene promoters (28). Some of these nucleoside analogues have been used in the treatment of myelodysplastic syn¬drome and acute myeloid leukemia (29).
The DNMT family of enzymes including DNMT1, DNM3A, and DNM3B are involved in catalyzing DNA methylation within the human genome. The transfer of a methyl group to DNA at position C5 of cytosine holds a significant role in gene regulation. This epigenetic modification is involved in the regulation of several gene functions including X-chromosome inactivation, genomic imprinting and silencing of proviral elements and retrotransposons (30). Abnormal DNA methylation, especially in the promoter regions of TSGs, alters gene expression and can facilitate human tumorigenesis. DNMT1 is the major DNMT in adult cells acting on hemimethylated CpG substrates and is involved in the maintenance of genomic DNA methylation during DNA repli-cation (31, 32).
Our findings showed an increase in the expression of the CDH1 and DNMT1 genes in APL patients compared to healthy controls. Using MSP, we observed that the promoter region of CDH1 and DNMT1 genes was un-methylated in most of the APL patients (12/15) and all of patients, respectively. These findings corroborate results from previous studies examining different types of cancers. Here are the studies on DNMT1 with the same results as ours Two paragraphs have the same citation. In colon cancer, it has been shown that DNMT1 expression is increased in comparison with normal mucosa (33). Additionally, DNMT1 activity was observed to increase with the advancement of tumor stage in colon and lung cancer (34, 35). A study examining hematologic malignancies found an increased expression of DNMT1 in 12 different leukemia samples including AML, ALL, and myelodysplastic syndrome (36). Robertson et al. have shown that DNMT1, DNM3A, and DNM3B are up-regulated in many different malignancies, such as bladder, colon, kidney, and pancreatic cancers (37). The overexpression of all three DNMTs has also been observed in many tumor cell lines (38).
Previous work examining the expression of DNMTs and the methylation pattern of TSGs in human pituitary adenomas found both overexpression of DNMTs and hypermethylation of the tumor suppressors, RASSF1A, CDH13, CDH1, and CDKN2A within the patient tumor samples. The high-methylation tumors had a higher frequency of DNMT1 and DNMT3A expression than the low-methylation tumors, p = 0.021 and 0.023, respectively. These findings support a potential role for DNMT1 and DNMT3A in the abnormal methylation of TSGs, contributing to the development of adenoma tissue invasion. This data suggests that targeting DNMTs may be an effective therapeutic approach for the treatment of invasive pituitary adenomas (39).
Previous work by Mizuno et al. explored how the abnormal hypermethylation of TSGs is involved in leukemia. In this study, the role of DNMT overexpression in the methylation of the cell cycle regulator p15(INAK4B) promoter region was investigated in patients with AML and chronic myelogenous leukemia (CML). They observed p15(INAK4B) to be methylated in 24 of the 33 (72%) AML cases. Interestingly, the AML cases with methylated p15(INAK4B) tended to express higher levels of DNMT1 and DNMT3B, compared to the cases with unmethylated p15(INAK4B). It was also shown that CML cells had an increased expression of DNMT1, DNMT3A, and DNMT3B in the acute phase in comparison to their levels in normal bone marrow cells. It was also found that these genes were increased in the leukemia cell lines, HL60, KU812, and K562s, compared to those in undergoing normal hematopoiesis. These findings suggest that overexpression of DNMTs may be involved in the leukemic transformation (40).
A study examining the alterations in DNMT1 promoter methylation and expression in patients with acute lymphoblastic leukemia (ALL) found that the patients with either B- or T-cell ALL showed no methylation of the DNMT1 gene. However, the methylation pattern of the pre-B ALL patients and control group showed partial promoter methylation. These findings suggest that determining the overall DNA methylation pattern may offer a means of predicting a patient’s prognosis and treatment response (41).
In a separate study, the role of DNMT1 on gene methylation status and cell proliferation was examined in RPMI-8226 human multiple myeloma (MM) cells. They observed that silencing the DNMT1 gene with siRNAs lead to a decrease in cell proliferation compared to the controls. The expression of B-cell lymphoma 2 and nuclear factor κB proteins were also significantly reduced. Furthermore, DNMT1 silencing also resulted in reduced methylation of the TSGs, suppressor of cytokine signaling 1 and p16. Their findings suggest that the silencing of the DNMT1 gene may be a promising strategy in the development of novel MM treatment methods (42).
The upregulation of DNMT1 gene in the above studies and our research represent the decisive role of this enzyme in scilencing genes which should be activated in normal tissues. As a result, it seems that decreasing DNMT1 gene expression would be of help in APL patients. (The analysis of other findings with respect to ours.)
A connection between the aberrant expression of E-cadherin and the development of metastasis in cancers has been previously reported. Studies have shown that abnormal E-cadherin expression is associated with cancer cell invasion and more advanced tumor staging for lung (43), prostate (44, 45), gastric (46), colorectal (47), and breast (48-50) cancer. Evidence suggests that when breast cancer cells reach distant sites, they may re-express E-cadherin protein (51). Previous research has revealed that the metastatic deposits from invasive ductal breast carcinomas expressed E-cadherin, and the level of expression was equal to or stronger than that of the main tumor. Indeed, by studying E- cadherin expression in nodal breast cancer metastases, Bukholm and colleagues found that 19 of 20 lymph node metastases strongly expressed the E-cadherin protein (52). E-cadherin re-expression facilitates intercellular adhesion and may enable malignant cells to form a metastatic deposit. The normal expression or re-expression of E-cadherin in cancer metastases appears to be similar in breast cancer and prostate cancer (51). Rubin and colleagues studied a large group of patients with prostate cancer including 77 distant metastases and detected normal E-cadherin expression in 90% of these cases (53).
The upregulation of CDH1 was also observed in a study examining Schwannomas and grade I meningiomas which are non-metastatic neoplasms. In this study it was shown that PDGFD, CDH1 and SLIT2 were upregulated in meningiomas and schwannomas when compared to their respective healthy tissues. Considering these results, it was suggested that these genes should be considered as targets in potential treatment procedures (54).
A study on amelobelastomas, which are a group of benign, locally aggressive, recurrent tumors characterized by their slow and infiltrative growth, showed that there was a strong relationship between the expression of CDH1 and tumor size and tumor regrowth. In order for potential aggressiveness of ameloblastoma variants to be determined, the assessment of the expression of E-cadherin and syndecan-1 is important. Future studies need to be conducted in order to understand how the expression of these genes is related to tumor aggressiveness (55). The up-regulation of CDH1 gene expression in the all mentioned works and, in our study, may present a role of this gene in cancer metastasis.
Our findings showed the expression of DNMT1 and CDH1 genes to be upregulated in patients with APL. We observed that 80% of the patient samples had an un-methylated status in the CDH1 gene promoter and the rest of the samples showed partial methylation. The un-methylation status of the DNMT1 gene promoter occurred in 100% of samples. As a result, CDH1 gene expression may be controlled by other mechanisms in APL. The upregulation of the DNMT1 gene in this study and in various cancers suggests that this enzyme has a critical role in silencing some genes who’s activation is important in healthy tissues. Given this, the reduction in DNMT1 gene by drugs or other mechanisms may serve as an effective treatment strategy for APL. The upregulation of CDH1 gene appears to indicate the principal role of this gene in cancer metastasis. In our work the expression of CDH1 (Ecad) was upregulated. However, further research with more samples is required to determine the role of CDH1 in the pathogenesis of APL. Moreover, investigation of other regulatory mechanisms of CDH1 in APL is needed. Since MSP technique is qualitative, it is suggested to make use of other techniques, Medip and Cobra for instance, in order to investigate the amount of methylation present in the CDH1 and DNMT1 genes in APL blood samples or cell lines.
Acknowledgements
We would like to show our gratitude to Qazvin University of Medical Sciences for supporting this work financially.
References
- 1.Magliulo D, Bernardi R, Messina S. Lysine-Specific Demethylase 1A as a Promising Target in Acute Myeloid Leukemia. Front Oncol. 2018;8:255. doi: 10.3389/fonc.2018.00255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dohner H, Weisdorf DJ, Bloomfield CD. Acute Myeloid Leukemia. N Engl J Med. 2015;373(12):1136–52. doi: 10.1056/NEJMra1406184. [DOI] [PubMed] [Google Scholar]
- 3.Alibhai SM, Leach M, Minden MD, Brandwein J. Outcomes and quality of care in acute myeloid leukemia over 40 years. Cancer. 2009;115(13):2903–11. doi: 10.1002/cncr.24373. [DOI] [PubMed] [Google Scholar]
- 4.Menzin J, Lang K, Earle CC, Kerney D, Mallick R. The outcomes and costs of acute myeloid leukemia among the elderly. Arch Intern Med. 2002;162(14):1597–603. doi: 10.1001/archinte.162.14.1597. [DOI] [PubMed] [Google Scholar]
- 5.Maali A, Ferdosi-Shahandashti E, Azad M. Drug Switching, a Creative Approach to Leukemia Therapy. IJBC. 2019;11(3):111–112. [Google Scholar]
- 6.Sun Y, Chen BR, Deshpande A. Epigenetic Regulators in the Development, Maintenance, and Therapeutic Targeting of Acute Myeloid Leukemia. Front Oncol. 2018;8:41. doi: 10.3389/fonc.2018.00041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Azad M, Kaviani S, Soleymani M, Nourouzinia M, Hajifathali A. Common polymorphism’s analysis of thiopurine S-methyltransferase (TPMT) in Iranian population. Yakhteh. 2009;11(3):311–316. [Google Scholar]
- 8.Bennett JM, Catovsky D, Daniel MT, Flandrin G, Galton DA, Gralnick HR, et al. Proposed revised criteria for the classification of acute myeloid leukemia. A report of the French-American-British Cooperative Group. Ann Intern Med. 1985;103(4):620–5. doi: 10.7326/0003-4819-103-4-620. [DOI] [PubMed] [Google Scholar]
- 9.Zhang L, Samad A, Pombo-de-Oliveira MS, Scelo G, Smith MT, Feusner J, et al. Global characteristics of childhood acute promyelocytic leukemia. Blood Rev. 2015;29(2):101–25. doi: 10.1016/j.blre.2014.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lo-Coco F, Ammatuna E, Montesinos P, Sanz MA. Acute promyelocytic leukemia: recent advances in diagnosis and management. Semin Oncol. 2008;35(4):401–9. doi: 10.1053/j.seminoncol.2008.04.010. [DOI] [PubMed] [Google Scholar]
- 11.Huang ME, Ye YC, Chen SR, Chai JR, Lu JX, Gu LJ, et al. Use of all-trans retinoic acid in the treatment of acute promyelocytic leukemia. Blood. 1988;72(2):567–72. [PubMed] [Google Scholar]
- 12.Calleja EM, Warrell RP. Differentiating agents in pediatric malignancies: all-trans-retinoic acid and arsenic in acute promyelocytic leukemia. Curr Oncol Rep. 2000;2(6):519–23. doi: 10.1007/s11912-000-0105-x. [DOI] [PubMed] [Google Scholar]
- 13.Ley TJ, Miller C, Ding L, Raphael BJ, Mungall AJ, Robertson A, et al. Genomic and epigenomic landscapes of adult de novo acute myeloid leukemia. N Engl J Med. 2013;368(22):2059–74. doi: 10.1056/NEJMoa1301689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sahmani M, Sakhinia E, Farzadi L, Najafipour R, Darabi M, Mehdizadeh A, et al. Two common polymorphisms in the peroxisome proliferator-activated receptor gamma gene may improve fertilization in IVF. Reprod Biomed Online. 2011;23(3):355–60. doi: 10.1016/j.rbmo.2011.05.005. [DOI] [PubMed] [Google Scholar]
- 15.Taby R, Issa JP. Cancer epigenetics. CA Cancer J Clin. 2010;60(6):376–92. doi: 10.3322/caac.20085. [DOI] [PubMed] [Google Scholar]
- 16.Azarkeivan A, Ahmadi MH, Zolfaghari S, Shaiegan M, Ferdowsi S, Rezaei N. RBC alloimmunization and double alloantibodies in thalassemic patients. Hematology. 2015;20(4):223–7. doi: 10.1179/1607845414Y.0000000189. [DOI] [PubMed] [Google Scholar]
- 17.Ghorban K, Shanaki M, Mobarra N, Azad M, Asadi J, Pakzad R, et al. Apolipoproteins A1, B, and other prognostic biochemical cardiovascular risk factors in patients with beta-thalassemia major. Hematology. 2016;21(2):113–20. doi: 10.1179/1607845415Y.0000000016. [DOI] [PubMed] [Google Scholar]
- 18.Esteller M. Epigenetics in cancer. N Engl J Med. 2008;358(11):1148–59. doi: 10.1056/NEJMra072067. [DOI] [PubMed] [Google Scholar]
- 19.Galm O, Herman JG, Baylin SB. The fundamental role of epigenetics in hematopoietic malignancies. Blood Rev. 2006;20(1):1–13. doi: 10.1016/j.blre.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 20.Azad M, Kaviani S, Noruzinia M, Mortazavi Y, Mobarra N, Alizadeh S, et al. Gene Expression Status and Methylation Pattern in Promoter of P15INK4b and P16INK4a in Cord Blood CD34 (+) Stem Cells. Iran J Basic Med Sci. 2013;16(7):822–8. [PMC free article] [PubMed] [Google Scholar]
- 21.Maali A, Atashi A, Ghaffari S, Kouchaki R, Abdolmaleki F, Azad M. A Review on Leukemia and iPSC Technology: Application in Novel Treatment and Future. Curr Stem Cell Res Ther. 2018;13(8):665–675. doi: 10.2174/1574888X13666180731155038. [DOI] [PubMed] [Google Scholar]
- 22.Subramaniam D, Thombre R, Dhar A, Anant S. DNA methyltransferases: a novel target for prevention and therapy. Front Oncol. 2014;4:80. doi: 10.3389/fonc.2014.00080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Berx G, van Roy F. Involvement of members of the cadherin superfamily in cancer. Cold Spring Harb Perspect Biol. 2009;1(6):a003129. doi: 10.1101/cshperspect.a003129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Asa SL, Ezzat S. The pathogenesis of pituitary tumours. Nat Rev Cancer. 2002;2(11):836–49. doi: 10.1038/nrc926. [DOI] [PubMed] [Google Scholar]
- 25.Fan J, Yin WJ, Lu JS, Wang L, Wu J, Wu FY, et al. ER alpha negative breast cancer cells restore response to endocrine therapy by combination treatment with both HDAC inhibitor and DNMT inhibitor. J Cancer Res Clin Oncol. 2008;134(8):883–90. doi: 10.1007/s00432-008-0354-x. [DOI] [PubMed] [Google Scholar]
- 26.Graca I, Sousa EJ, Baptista T, Almeida M, Ramalho-Carvalho J, Palmeira C, et al. Anti-tumoral effect of the non-nucleoside DNMT inhibitor RG108 in human prostate cancer cells. Curr Pharm Des. 2014;20(11):1803–11. doi: 10.2174/13816128113199990516. [DOI] [PubMed] [Google Scholar]
- 27.Song SH, Han SW, Bang YJ. Epigenetic-based therapies in cancer: progress to date. Drugs. 2011;71(18):2391–403. doi: 10.2165/11596690-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 28.Gabbara S, Bhagwat AS. The mechanism of inhibition of DNA (cytosine-5-)-methyltransferases by 5-azacytosine is likely to involve methyl transfer to the inhibitor. Biochem J. 1995;307(Pt 1):87–92. doi: 10.1042/bj3070087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sigalotti L, Fratta E, Coral S, Cortini E, Covre A, Nicolay HJ, et al. Epigenetic drugs as pleiotropic agents in cancer treatment: biomolecular aspects and clinical applications. J Cell Physiol. 2007;212(2):330–44. doi: 10.1002/jcp.21066. [DOI] [PubMed] [Google Scholar]
- 30.Baylin SB, Ohm JE. Epigenetic gene silencing in cancer - a mechanism for early oncogenic pathway addiction?. Nat Rev Cancer. 2006;6(2):107–16. doi: 10.1038/nrc1799. [DOI] [PubMed] [Google Scholar]
- 31.Bestor TH. The DNA methyltransferases of mammals. Hum Mol Genet. 2000;9(16):2395–402. doi: 10.1093/hmg/9.16.2395. [DOI] [PubMed] [Google Scholar]
- 32.Szyf M, Pakneshan P, Rabbani SA. DNA methylation and breast cancer. Biochem Pharmacol. 2004;68(6):1187–97. doi: 10.1016/j.bcp.2004.04.030. [DOI] [PubMed] [Google Scholar]
- 33.el-Deiry WS, Nelkin BD, Celano P, Yen RW, Falco JP, Hamilton SR, et al. High expression of the DNA methyltransferase gene characterizes human neoplastic cells and progression stages of colon cancer. Proc Natl Acad Sci U S A. 1991;88(8):3470–4. doi: 10.1073/pnas.88.8.3470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Belinsky SA, Nikula KJ, Baylin SB, Issa JP. Increased cytosine DNA-methyltransferase activity is target-cell-specific and an early event in lung cancer. Proc Natl Acad Sci U S A. 1996;93(9):4045–50. doi: 10.1073/pnas.93.9.4045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Issa JP, Vertino PM, Wu J, Sazawal S, Celano P, Nelkin BD, et al. Increased cytosine DNA-methyltransferase activity during colon cancer progression. J Natl Cancer Inst. 1993;85(15):1235–40. doi: 10.1093/jnci/85.15.1235. [DOI] [PubMed] [Google Scholar]
- 36.Melki JR, Warnecke P, Vincent PC, Clark SJ. Increased DNA methyltransferase expression in leukaemia. Leukemia. 1998;12(3):311–316. doi: 10.1038/sj.leu.2400932. [DOI] [PubMed] [Google Scholar]
- 37.Robertson KD, Uzvolgyi E, Liang G, Talmadge C, Sumegi J, Gonzales FA, et al. The human DNA methyltransferases (DNMTs) 1, 3a and 3b: coordinate mRNA expression in normal tissues and overexpression in tumors. Nucleic Acids Res. 1999;27(11):2291–8. doi: 10.1093/nar/27.11.2291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xie S, Wang Z, Okano M, Nogami M, Li Y, He WW, et al. Cloning, expression and chromosome locations of the human DNMT3 gene family. Gene. 1999;236(1):87–95. doi: 10.1016/s0378-1119(99)00252-8. [DOI] [PubMed] [Google Scholar]
- 39.Ma HS, Wang EL, Xu WF, Yamada S, Yoshimoto K, Qian ZR, et al. Overexpression of DNA (Cytosine-5)-Methyltransferase 1 (DNMT1) And DNA (Cytosine-5)-Methyltransferase 3A (DNMT3A) Is Associated with Aggressive Behavior and Hypermethylation of Tumor Suppressor Genes in Human Pituitary Adenomas. Med Sci Monit. 2018;24:4841–4850. doi: 10.12659/MSM.910608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mizuno S, Chijiwa T, Okamura T, Akashi K, Fukumaki Y, Niho Y, et al. Expression of DNA methyltransferases DNMT1, 3A, and 3B in normal hematopoiesis and in acute and chronic myelogenous leukemia. Blood. 2001;97(5):1172–9. doi: 10.1182/blood.v97.5.1172. [DOI] [PubMed] [Google Scholar]
- 41.Rahmani T, Azad M, Chahardouli B, Nasiri H, Vatanmakanian M, Kaviani S, et al. Patterns of DNMT1 Promoter Methylation in Patients with Acute Lymphoblastic Leukemia. Int J Hematol Oncol Stem Cell Res. 2017;11(3):172–177. [PMC free article] [PubMed] [Google Scholar]
- 42.Zhou W, Chen H, Hong X, Niu X, Lu Q. Knockdown of DNA methyltransferase-1 inhibits proliferation and derepresses tumor suppressor genes in myeloma cells. Oncol Lett. 2014;8(5):2130–2134. doi: 10.3892/ol.2014.2481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shimoyama Y, Hirohashi S, Hirano S, Noguchi M, Shimosato Y, Takeichi M, et al. Cadherin cell-adhesion molecules in human epithelial tissues and carcinomas. Cancer Res. 1989;49(8):2128–33. [PubMed] [Google Scholar]
- 44.Umbas R, Isaacs WB, Bringuier PP, Schaafsma HE, Karthaus HF, Oosterhof GO, et al. Decreased E-cadherin expression is associated with poor prognosis in patients with prostate cancer. Cancer Res. 1994;54(14):3929–33. [PubMed] [Google Scholar]
- 45.Umbas R, Isaacs WB, Bringuier PP, Xue Y, Debruyne FM, Schalken JA. Relation between aberrant alpha-catenin expression and loss of E-cadherin function in prostate cancer. Int J Cancer. 1997;74(4):374–7. doi: 10.1002/(sici)1097-0215(19970822)74:4<374::aid-ijc2>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 46.Mayer B, Johnson JP, Leitl F, Jauch KW, Heiss MM, Schildberg FW, et al. E-cadherin expression in primary and metastatic gastric cancer: down-regulation correlates with cellular dedifferentiation and glandular disintegration. Cancer Res. 1993;53(7):1690–5. [PubMed] [Google Scholar]
- 47.Dorudi S, Hanby AM, Poulsom R, Northover J, Hart IR. Level of expression of E-cadherin mRNA in colorectal cancer correlates with clinical outcome. Br J Cancer. 1995;71(3):614–616. doi: 10.1038/bjc.1995.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Oka H, Shiozaki H, Kobayashi K, Inoue M, Tahara H, Kobayashi T, et al. Expression of E-cadherin cell adhesion molecules in human breast cancer tissues and its relationship to metastasis. Cancer Res. 1993;53(7):1696–701. [PubMed] [Google Scholar]
- 49.Palacios J, Benito N, Pizarro A, Suarez A, Espada J, Cano A, et al. Anomalous expression of P-cadherin in breast carcinoma. Correlation with E-cadherin expression and pathological features. Am J Pathol. 1995;146(3):605–612. [PMC free article] [PubMed] [Google Scholar]
- 50.Rasbridge SA, Gillett CE, Sampson SA, Walsh FS, Millis RR. Epithelial (E-) and placental (P-) cadherin cell adhesion molecule expression in breast carcinoma. J Pathol. 1993;169(2):245–50. doi: 10.1002/path.1711690211. [DOI] [PubMed] [Google Scholar]
- 51.Kowalski PJ, Rubin MA, Kleer CG. E-cadherin expression in primary carcinomas of the breast and its distant metastases. Breast Cancer Res. 2003;5(6):R217–22. doi: 10.1186/bcr651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bukholm IK, Nesland JM, Borresen-Dale AL. Re-expression of E-cadherin, alpha-catenin and beta-catenin, but not of gamma-catenin, in metastatic tissue from breast cancer patients. J Pathol. 2000;190(1):15–9. doi: 10.1002/(SICI)1096-9896(200001)190:1<15::AID-PATH489>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 53.Rubin MA, Mucci NR, Figurski J, Fecko A, Pienta KJ, Day ML. E-cadherin expression in prostate cancer: a broad survey using high-density tissue microarray technology. Hum Pathol. 2001;32(7):690–7. doi: 10.1053/hupa.2001.25902. [DOI] [PubMed] [Google Scholar]
- 54.Torres-Martin M, Lassaletta L, Isla A, De Campos JM, Pinto GR, Burbano RR, et al. Global expression profile in low grade meningiomas and schwannomas shows upregulation of PDGFD, CDH1 and SLIT2 compared to their healthy tissue. Oncol Rep. 2014;32(6):2327–2334. doi: 10.3892/or.2014.3526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Carreon-Burciaga RG, Gonzalez-Gonzalez R, Molina-Frechero N, Lopez-Verdin S, Pereira-Prado V, Bologna-Molina R, et al. Differences in E-Cadherin and Syndecan-1 Expression in Different Types of Ameloblastomas. Anal Cell Pathol (Amst). 2018;2018:9392632. doi: 10.1155/2018/9392632. [DOI] [PMC free article] [PubMed] [Google Scholar]


