Fig. 1. Classical paradigm of GPCR activation and biased signaling.
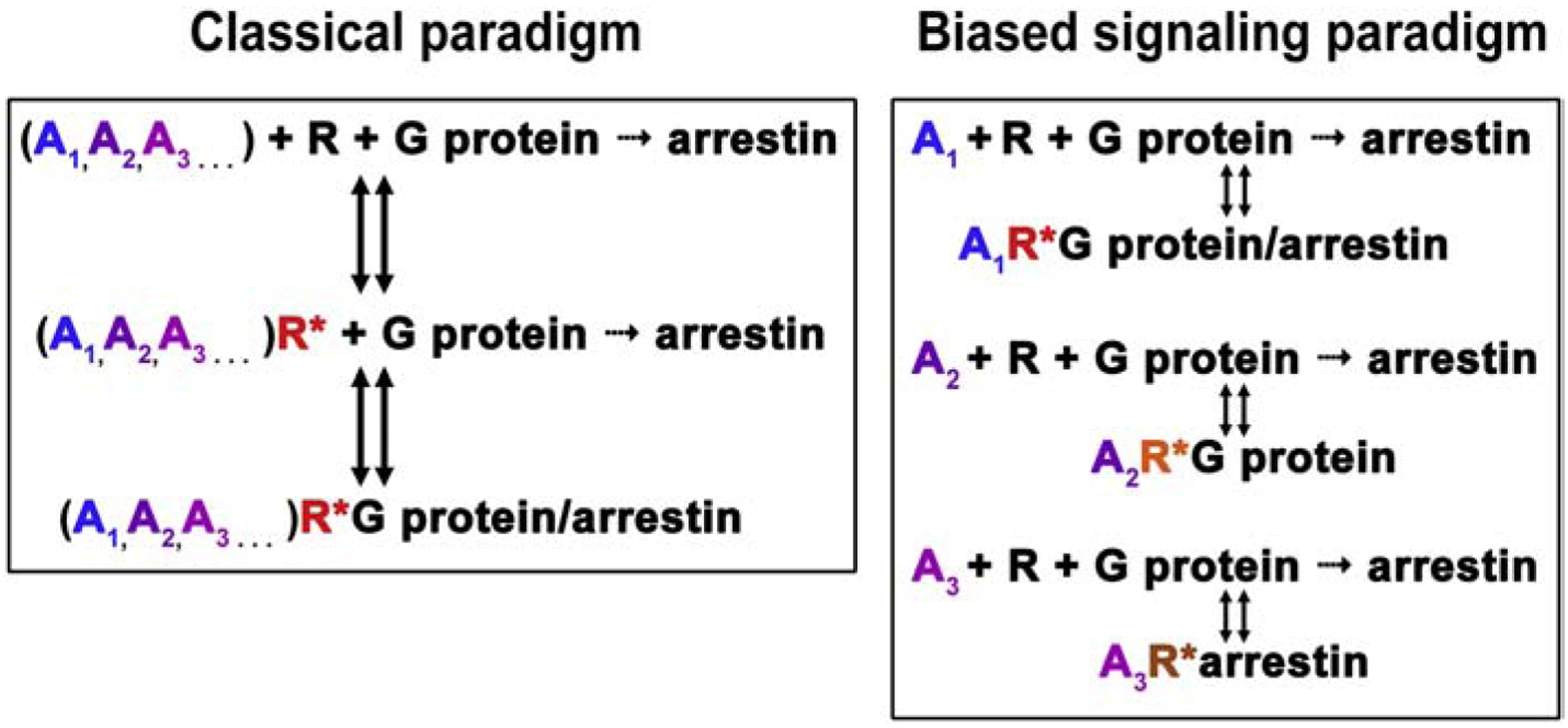
Classical extended ternary complex model (Samama, et al., 1993) of GPCR activation was based on the assumption that receptors have only two conformations, active (R*) and inactive (R). Receptors activated by any agonist (shown as differently colored A1, A2, A3) were thought to couple to G proteins, become phosphorylated by GRKs, and then bind arrestins. Arrestin binding terminated G protein-mediated signaling (Carman & Benovic, 1998). The paradigm of biased signaling is based on the idea that different agonists induce distinct active receptor conformations (shown as differently colored R*), some of which are equally good for G proteins and arrestins, whereas others differentially affect receptor interactions with these signaling partners, demonstrating bias towards G proteins or arrestins.
