Abstract
Purpose
There are very little data available comparing outcomes of intensity-modulated proton therapy (IMPT) to intensity-modulated radiation therapy (IMRT) in patients with locally advanced NSCLC (LA-NSCLC).
Methods
Seventy-nine consecutively treated patients with LA-NSCLC underwent definitive IMPT (n = 33 [42%]) or IMRT (n = 46 [58%]) from 2016 to 2018 at our institution. Survival rates were calculated using the Kaplan-Meier method and compared with the log-rank test. Acute and subacute toxicities were graded based on Common Terminology Criteria for Adverse Events, version 4.03.
Results
Median follow-up was 10.5 months (range, 1-27) for all surviving patients. Most were stage III (80%), received median radiation therapy (RT) dose of 60 Gy (range, 45-72), and had concurrent chemotherapy (65%). At baseline, the IMPT cohort was older (76 vs 69 years, P < .01), were more likely to be oxygen-dependent (18 vs 2%, P = .02), and more often received reirradiation (27 vs 9%, P = .04) than their IMRT counterparts. At 1 year, the IMPT and IMRT cohorts had similar overall survival (68 vs 65%, P = .87), freedom from distant metastasis (71 vs 68%, P = .58), and freedom from locoregional recurrence (86 vs 69%, P = .11), respectively. On multivariate analyses, poorer pulmonary function and older age were associated with grade +3 toxicities during and 3 months after RT, respectively (both P ≤ .02). Only 5 (15%) IMPT and 4 (9%) IMRT patients experienced grade 3 or 4 toxicities 3 months after RT (P = .47). There was 1 treatment-related death from radiation pneumonitis 6 months after IMRT in a patient with idiopathic pulmonary fibrosis.
Conclusions
Compared with IMRT, our early experience suggests that IMPT resulted in similar outcomes in a frailer population of LA-NSCLC who were more often being reirradiated. The role of IMPT remains to be defined prospectively.
Introduction
Lung cancer remains the leading cause of cancer-related death worldwide.1 Non-small cell lung cancer (NSCLC) accounts for approximately 85% of all new cases, with a poor 5-year survival rate of 15% to 30%.2 In the locally advanced setting, which accounts for approximately one-third of patients with newly diagnosed NSCLC, combined modality therapy is the standard of care.3, 4, 5, 6, 7
Patients receiving definitive chemoradiation for locally advanced NSCLC (LA-NSCLC) are at significant risk for developing treatment-related thoracic toxicities. In particular, elderly patients with poor performance status or existing cardiopulmonary comorbidities are at greatest risk for treatment-related side effects and inferior outcomes.8, 9, 10, 11 Severe (grade ≥3) toxicities are quite common. In one large trial of concurrent chemoradiation (cisplatin, etoposide, and 60 Gy in 30 daily fractions of thoracic radiation therapy), 53% had grade ≥3 acute nonhematologic toxicities.12 The most common severe toxicities were myelosuppression followed by nausea and vomiting, pulmonary toxicities, and esophagitis. In addition, RTOG 0617 demonstrated cardiac radiation dose (heart V40) as an independent predictor of overall survival.13 Therefore, reducing radiation-induced toxicities is important.14 In the reirradiation setting, the risk of treatment-related toxicities is potentially worse.15, 16, 17
The use of proton beam therapy (PBT), as a result, is an attractive option especially for frailer patients who may otherwise be deemed unfit for conventional photon-based radiotherapies. Conventional photon-based radiation therapy (RT) is limited in its ability to maximally spare normal cardiopulmonary structures and simultaneously deliver high target doses. PBT has the ability to stop immediately in the beam path's length after the tumor volume is fully irradiated but before traveling through critical normal structures distal to the target volume owing to its Bragg peak phenomenon. Intensity-modulated proton therapy (IMPT) has dosimetric advantages over 3-dimensional (3-D) protons and all photon options.18, 19, 20 Despite higher prescription doses in the PBT cohorts, single institution series have demonstrated reduced rates of severe treatment-related toxicities compared with photon RT.21, 22 A phase II study of concurrent chemotherapy with PBT in unresectable stage III NSCLC demonstrated comparable survival with reduced toxicities compared with RTOG 0617.23 However, almost all prior series of PBT in LA-NSCLC have used passively scattered PBT as opposed to IMPT, which uses an active scanning delivery system, a more advanced form of PBT.24 As proton beam centers with IMPT capabilities are increasing both nationally and internationally, there are significantly more LA-NSCLC patients who will be treated with IMPT in the future.
As a result, studies are urgently needed to describe the outcomes of IMPT in LA-NSCLC. In this study, we compare the early outcomes of 79 consecutive patients with LA-NSCLC treated with IMPT versus intensity-modulated radiation therapy (IMRT).
Methods and Materials
This was an institutional review board–approved study. Between March 2016 and June 2018, 79 patients with LA-NSCLC were treated with definitive IMPT (N = 33) or IMRT (N = 46), with or without concurrent chemotherapy at the Mayo Clinic in Arizona. The following were used as inclusion criteria: (1) ≥18 years old; (2) biopsy-proven or radiologic diagnosis of primary or recurrent NSCLC (all primary diagnoses were biopsy-proven); (3) receipt of IMPT or IMRT with curative intent, ie, >45 Gy with fractionated treatments. Patients with prior thoracic RT were purposefully included in this study as it is a common indication for PBT.
All patients underwent a complete history and physical examination by both a medical and radiation oncologist. All patients received either computed tomography (CT) or positron emission tomography imaging; in our practice, the majority of them received positron emission tomography/CT. A magnetic resonance imaging of the brain was also required as part of staging. The vast majority of diagnoses were biopsy proven (except for 7 cases with recurrent disease). Tumor staging was based on the seventh edition of the American Joint Committee on Cancer staging system. Before treatment, each patient underwent multidisciplinary evaluation, and the treatment methods were agreed upon by all involved providers including the choice of RT modality (IMPT vs IMRT). Factors that influenced choice of treatment modality include older age, poor pulmonary function, poor functional status, tumor location, tumor size, and prior radiation therapy.
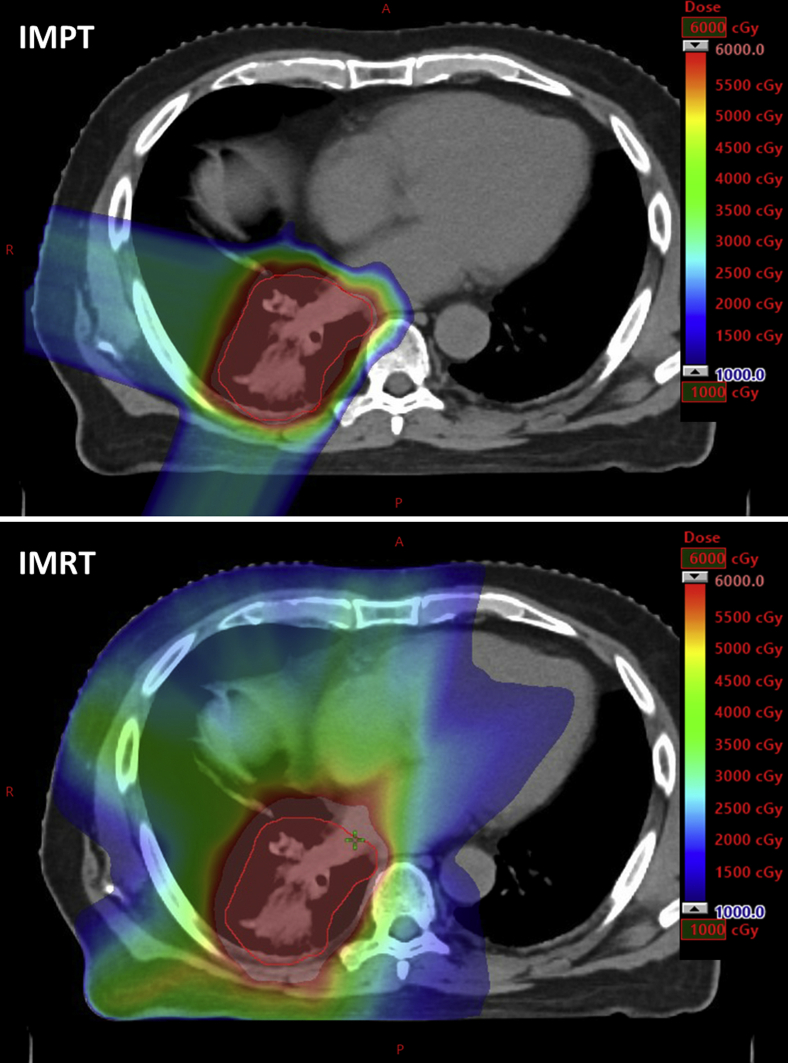
All patients were immobilized in the supine position with their arms above their head using Orfit board (Orfit Industries, Wijnegem, Belgium) and thermoplastic masks. High-resolution 4-dimensional CT (4D-CT) simulation images without contrast were obtained to assess respiratory motion. All patients were treated to radiographically involved sites of disease without prophylactic nodal coverage. The dosimetric advantages of IMPT were demonstrated for a patient treated in the present study (Fig 1).
Figure 1.
An axial comparison of intensity-modulated proton therapy (IMPT; top) versus intensity-modulated radiation therapy (IMRT; bottom) dose distribution. The optimization target volume (IMPT) and planning target volume (IMRT) are contoured in red. This is a 74-year-old man in the present study with stage IIIB adenocarcinoma of the right lower lobe lung who received (top) 60 Gy in 30 fractions with concurrent and consolidative carboplatin and paclitaxel. IMPT was delivered using 3 fields (posterior, lateral, and right posterior oblique). At 9 months after completion of IMPT, the patient demonstrated no evidence of new or progressive disease.
IMPT was delivered using a pencil-beam active-scanning delivery system (Hitachi ProBeat, Tokyo, Japan). An internal gross-tumor target volume (IGTV) was defined to encompass an envelope in space containing the gross disease in all phases of respiratory motion based on 4D-CT treatment planning images. A 5- to 10-mm expansion was added to the IGTV to account for potential microscopic spread which was designated as internal target volume (ITV). An optimization target volume, designed to account for daily setup uncertainties, was typically defined as a 5-mm isotropic expansion based on the ITV, which could be modified based on beam angles and patient-specific anatomic considerations. A scanning target volume, designed to guide initial IMPT spot placement, was defined as the optimization target volume with a 7-mm isotropic expansion. IMPT plans were generated by optimizing radiation dose to the ITV on averaged 4D-CTs with possible density override to IGTV. Typically, 2 to 3 treatment fields were used. Beam angles were determined by experienced dosimetrists, physicists, and in-house developed software.25 An assumed relative biologic effectiveness of 1.1 for protons was used for all IMPT dose calculations. Two verification plans were generated by recalculating the dose on expiration and inhalation 4D-CT phases (without the density override) to evaluate the effect of respiratory motion. The original plan was adjusted until the verification and original plan dose distributions met all the required dose volume constraints, plan robustness quantification thresholds, and RT dose prescription criteria. In the aforementioned scenarios, plan robustness was rigorously considered in all cases. Interplay effect was also assessed by in-house developed software to make sure that D95% of the target would be at least 95% of the prescription dose.26, 27
IMRT was delivered using standard coplanar techniques, by either fixed beam angles, or more contemporarily, volumetric-based modulated RT. If necessary, respiratory-gated or breath-hold radiation therapy techniques were used to accommodate tumor motion; however, the majority of patients were treated with free-breathing. The GTV, IGTV, and ITV were defined identically compared with our IMPT practice (as discussed earlier). A planning target volume was defined as the ITV with typically a 5-mm isotropic expansion. Plan robustness and interplay effects were not considered in IMRT plans. The readers can find the dosimetric details of IMPT and IMRT treatment planning in this study by our group.19 Acute (during RT), subacute toxicities (3 months after completion of RT), and chronic (6 months after completion of RT) were graded and recorded according to the Common Terminology Criteria for Adverse Events, version 4.03.
Statistical analysis
As part of a prospective registry at the department, patient, tumor, and treatment characteristics were extracted and analyzed with comparative statistics and Fisher exact testing. Overall survival (OS), freedom from distant metastasis (FFDM), and freedom from locoregional recurrence (FFLR) were retrospectively verified and calculated using the Kaplan-Meier method. OS was referenced from initiating RT. FFDM was defined as the time from initiating RT to development of any distant metastasis or last follow-up. FFLR was defined as the time from initiating RT to any locoregional recurrence or last follow-up. Locoregional recurrence was defined as recurrence within the radiation field or regional nodal stations (surrounding lymphatics). All other recurrences were considered as distant metastases. Univariate and multivariate Cox regressions were used to create the final models. A P value of less than .05 was considered statistically significant, and all hypothesis testing was 2-tailed. The log-rank test was used to compare Kaplan-Meier projections. All analyses were performed in SAS version 9.4 (SAS Institute, Cary, NC).
Results
The median follow-up since initiation of RT for surviving patients was 10.5 months (range, 1-27). Median follow-up was 8.5 months for all patients. Patient, cancer, and treatment characteristics are listed in Table 1. The following cancer and treatment characteristics were not statistically different between the IMPT and IMRT cohorts: the majority of patients had stage III NSCLC (79%), the median RT dose was 60 Gy (range, 45-72; adjusted for relative biologic effectiveness) delivered with a median of 30 (range, 10-39) fractions, and 65% of patients received concurrent chemotherapy. Median daily dose was 2 Gy (range, 1.9-5) in the IMPT cohort and 2 Gy (range, 1.5-2) in the IMRT cohort. Of the 13 patients who received reirradiation, 2 (15%) received concurrent chemotherapy. All patients received RT with or without chemotherapy as curative intent. In addition, the presence of comorbidities, including stroke, kidney disease, hypertension, coronary artery disease, and diabetes mellitus, were not statistically different between the IMPT and IMRT cohorts. However, the IMPT cohort was older (median age: 75 vs 69 years, P < .01), more often were oxygen-dependent (18 vs 2%, P = .02), and more often received reirradiation (27 vs 9%, P = .04) than their IMRT counterpart. In addition, there were more patients with squamous cell carcinoma and fewer patients with adenocarcinoma histology in the IMPT cohort (P < .01; Table 1).
Table 1.
Patient, cancer, and treatment characteristics by treatment modality (n = 79)
| Characteristic | Treatment modality |
P Value | |
|---|---|---|---|
| IMPT n = 33 (%) | IMRT n = 46 (%) | ||
| Mean age at diagnosis, y (SD) | 75.8 (6) | 69.3 (11) | <.01 |
| Sex | .50 | ||
| Male | 20 (61) | 24 (52) | |
| ECOG PS | .11 | ||
| 0 | 6 (18) | 19 (41) | |
| 1 | 19 (58) | 18 (39) | |
| 2 | 7 (21) | 6 (13) | |
| 3 | 1 (3) | 3 (7) | |
| Smoking status | .77 | ||
| Nonsmoker | 5 (15) | 9 (20) | |
| Former/current smoker | 28 (85) | 37 (80) | |
| Stroke | .51 | ||
| Absent | 33 (100) | 44 (96) | |
| Present | 0 (0) | 2 (4) | |
| Kidney disease | .71 | ||
| Absent | 29 (88) | 42 (91) | |
| Present | 4 (12) | 4 (9) | |
| Hypertension | .25 | ||
| Absent | 16 (49) | 29 (63) | |
| Present | 17 (52) | 17 (40) | |
| Coronary artery disease | .24 | ||
| Absent | 25 (76) | 40 (87) | |
| Present | 8 (24) | 6 (13) | |
| Diabetes mellitus | .71 | ||
| Absent | 29 (88) | 42 (91) | |
| Present | 4 (12) | 4 (9) | |
| Oxygen-dependent COPD | .02 | ||
| Absent | 27 (82) | 45 (98) | |
| Present | 6 (18) | 1 (2) | |
| Histology | <.01 | ||
| Adenocarcinoma | 12 (36) | 32 (70) | |
| Squamous cell carcinoma | 19 (58) | 12 (26) | |
| Large cell/neuroendocrine | 2 (6) | 2 (4) | |
| AJCC stage III or higher | .40 | ||
| I | 3 (9) | 0 | |
| II | 3 (9) | 4 (9) | |
| IIIA | 17 (52) | 25 (54) | |
| IIIB | 7 (21) | 13 (28) | |
| IV | 3 (9) | 4 (9) | |
| Total radiation dose, Gy | .19 | ||
| Median (range) | 60 (45-72) | 60 (54-66) | |
| Mean (SD) | 58 (6) | 60 (1) | |
| Concurrent chemotherapy | .34 | ||
| No | 14 (42) | 14 (30) | |
| Yes | 19 (58) | 32 (70) | |
| Reirradiation | .04 | ||
| No | 24 (73) | 42 (91) | |
| Yes | 9 (27) | 4 (9) | |
Abbreviations: AJCC = American Joint Committee on Cancer; COPD = chronic obstructive pulmonary disease; IMPT = intensity-modulated proton therapy; IMRT = intensity-modulated radiation therapy; SD = standard deviation.
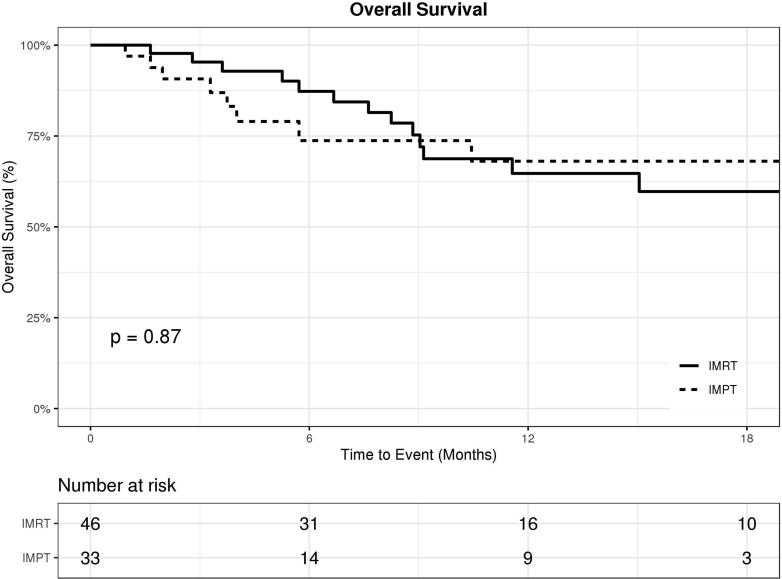
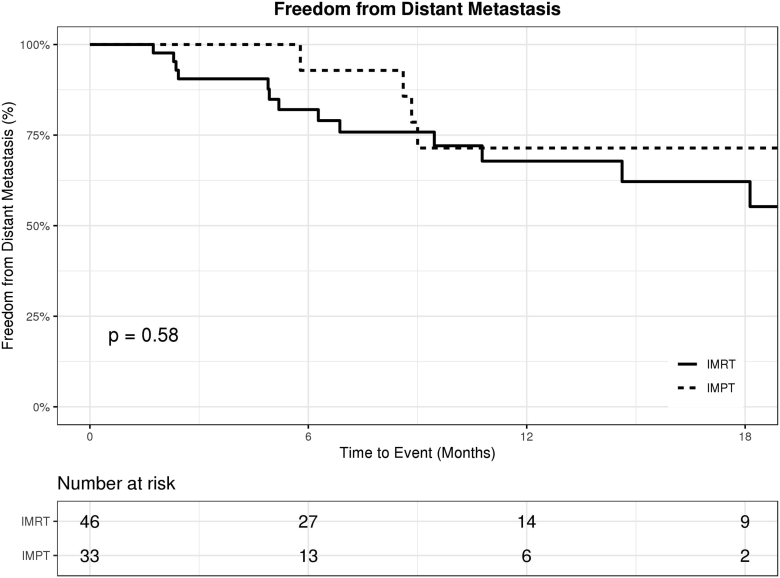
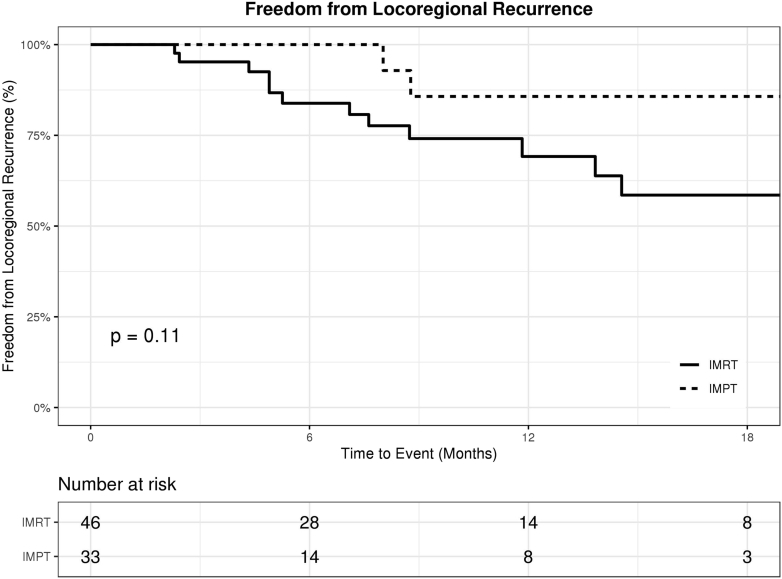
There were no significant differences between the IMPT and IMRT cohorts in terms of OS, FFDM, and FFLR. The 1-year OS rate was 68% (95% confidence interval [CI], 45%-83%) in the IMPT cohort, compared with 65% (95% CI, 46%-79%) in the IMRT cohort (P = .87; Fig 2). The 1-year FFDM rate was 71% (95% CI, 41%-88%) in the IMPT cohort, compared with 68% (95% CI, 49%-81%) in the IMRT cohort (P = .58; Fig 3). The 1-year FFLR rate was 86% (95% CI, 54%-96%) in the IMPT cohort, compared with 69% (95% CI, 49%-83%) in the IMRT cohort (P = .11; Fig 4). Univariate analyses are summarized in Table 2. Reirradiation was not significantly associated with OS, FFDM, FFLR, or toxicities. On multivariate analyses, Eastern Cooperative Oncology Group performance status of 2 or 3 versus 0 or 1 predicted worse OS (HR 7, P < .01).
Figure 2.
Kaplan-Meier curve for overall survival in patients treated with intensity-modulated proton therapy (IMPT; dotted) versus intensity-modulated radiation therapy (IMRT; solid).
Figure 3.
Freedom from distant metastasis in patients treated with intensity-modulated proton therapy (IMPT; dotted) versus intensity-modulated radiation therapy (IMRT; solid).
Figure 4.
Freedom from locoregional recurrence in patients treated with intensity-modulated proton therapy (IMPT; dotted) versus intensity-modulated radiation therapy (IMRT; solid).
Table 2.
Summary of univariate analyses for overall survival, freedom from distant metastasis, and freedom from locoregional recurrence (n = 79)
| Variable | OS |
FFDM |
FFLR |
|||
|---|---|---|---|---|---|---|
| HR (95% CI) | P Value | HR (95% CI) | P Value | HR (95% CI) | P Value | |
| Reirradiation (yes vs no) | 1.3 (0.5-3.5) | .62 | 0.7 (0.2-2.3) | .53 | 0.27 (0.03-2.1) | .21 |
| Concurrent Chemotherapy (yes vs no) | 0.9 (0.4-2.1) | .78 | 2.3 (0.8-6.7) | .11 | 1.8 (0.6-5.7) | .33 |
| ECOG (2 or 3 vs 0 or 1) | 2.9 (1.2-7.1) | .02 | 2.0 (0.6-6.2) | .24 | 0.4 (0.04-2.7) | .31 |
| Sex (female vs male) | 0.6 (0.2-1.5) | .26 | 0.7 (0.3-1.6) | .36 | 0.3 (0.1-1.0) | .06 |
| Age (per y) | 1.0 (1.0-1.1) | .08 | 0.95 (0.9-1.0) | .03 | 1.0 (0.9-1.0) | .11 |
| Received RT dose (per Gy) | 0.9 (0.8-1.0) | .06 | 1.0 (0.9-1.1) | 1.00 | 1.2 (1.0-1.3) | .01 |
| Smoking (Smoked vs never) | 0.89 (0.3-2.6) | .83 | 0.7 (0.2-2.2) | .56 | 0.4 (0.1-1.1) | .07 |
| Modality (IMPT vs IMRT) | 1.1 (0.4-2.6) | .87 | 0.7 (0.3-2.1) | .58 | 0.3 (0.1-1.4) | .13 |
| Kidney disease | 1.7 (0.5-5.7) | .41 | 1.6 (0.5-5.7) | .45 | 0.6 (0.1-4.9) | .66 |
| (yes vs no) | 1.4 (0.6-3.4) | .42 | 0.7 (0.3-1.9) | .50 | 0.3 (0.1-1.1) | .08 |
| Hypertension | ||||||
| (yes vs no) | 2.6 (0.9-7.1) | .07 | 2.2 (0.62-7.5) | .23 | 1.5 (0.3-6.7) | .60 |
| Diabetes | ||||||
| (yes vs no) | 1.8 (0.7-5.0) | .25 | 0.3 (0.04-2.6) | .29 | 0.4 (0.1-3.3) | .42 |
| CAD (yes vs no) | ||||||
Abbreviations: CAD = coronary artery disease; CI = confidence interval; FFDM = freedom from distant metastasis; FFLR = freedom from locoregional recurrence; HR = hazard ratio; IMPT = intensity-modulated proton therapy; IMRT = intensity-modulated radiation therapy; OS = overall survival; RT = radiation therapy.
Overall, both IMPT and IMRT were well tolerated. There were no significant differences in grade 3 or 4 subacute (3 months post-RT) toxicities between IMPT and IMRT (5 vs 4 patients, P = .47, respectively). Grade 3 subacute radiation pneumonitis rates were not statistically different between IMPT and IMRT (2 vs 1 patient, P = .56, respectively). Grade 3 subacute esophagitis rates were not statistically different between IMPT and IMRT (2 vs 0 patients, P = .56, respectively). Grade 3 subacute dyspnea rates were not statistically different between IMPT and IMRT (1 vs 3 patients, P = .64, respectively). There were no new grade 3 toxicities in either cohort at 6 months after completion of RT. Grade 4 toxicities were limited to dyspnea in one patient with a significant smoking history who received reirradiation alone to 50 Gy in 20 fractions with IMPT for a locally recurrent, obstructive, left upper lobe squamous cell carcinoma. He had previously received 60 Gy in 30 fractions with IMRT and concurrent chemotherapy (paclitaxel and carboplatin) 1 year before reirradiation. Four months after reirradiation, this patient developed life-threatening dyspnea with evidence of a large left hydropneumothorax as a result of a left upper lobe bronchopleural fistula; he did subsequently recover from this injury. This was presumed to be due to a combination of infection, reirradiation, and tumor recurrence. There was a single grade 5 toxicity. An elderly man developed diffuse radiation pneumonitis in the setting of idiopathic pulmonary fibrosis 6 months after IMRT. The patient died shortly thereafter despite appropriate hospital admission and pulmonary care. Using multivariate analyses and controlling for baseline toxicities, more severe chronic obstructive pulmonary disease was associated with any grade 3 or higher acute toxicities (odds ratio 8.6; 95% confidence interval, 1.3-57.5; P = .02), and older age was associated with any grade 3 or higher subacute toxicities (odds ratio 1.1; 95% CI, 1.0-1.3; P < .01).
Discussion
To our knowledge, this is the first comparative study of IMPT versus IMRT for patients with locally advanced primary or recurrent NSCLC. Despite our IMPT cohort containing functionally older patients, the early outcomes demonstrated that IMPT achieved similar clinical outcomes and toxicities compared with IMRT. This is important for 2 reasons. First, we treated tumors with the same doses as conventional photon-based RT (ie, IMRT) while reducing the exposure dose to surrounding organs at-risk and potentially improving toxicity profiles and disease outcome. Second, for patients with lung cancer, which is primarily a disease of the elderly, the treatment must become more tolerable especially with a majority with a smoking history, cardiopulmonary disease, and other comorbidities. With a rapidly aging population, there is an urgent need to define the role of PBT.
Concurrent chemoradiation and immunotherapy is the new standard of care for LA-NSCLC. Survival outcomes for LA-NSCLC are improving in the context of immunotherapy.7 The role of targeted therapy is not yet clearly defined in nonmetastatic NSCLC. However, during the time the patients in the present study were treated, chemoradiation was the standard of care. After chemoradiation, approximately one-third of patients develop local-regional recurrence, which may be amenable to reirradiation. One of the challenges in this population is seeking to minimize treatment-related toxicities. This is particularly challenging in elderly patients with cardiopulmonary comorbidities.
PBT has demonstrated encouraging clinical outcomes for LA-NSCLC with less toxicities than IMRT.21, 22, 23, 24 PBT can allow for dose escalation to the tumor volume and reduction in dose to normal tissues, although the current focus has been on decreasing unnecessary RT dose to healthy organs to improve outcome.28, 29, 30 Hoppe et al demonstrated that dose-escalated PBT delivering 74 to 80 Gy with 2 Gy per fraction with concurrent chemotherapy was well tolerated in a single-institution phase 2 trial for stage III NSCLC.31 PBT has demonstrated no differences in patterns of locoregional failure compared with IMRT.32 In the recently reported MDACC phase II series by Chang et al, concurrent chemotherapy with PBT for unresectable stage III NSCLC demonstrated comparable survival with reduced toxicities compared with the historical results from RTOG 0617.24 Zhu et al demonstrated that unfavorable-risk LA-NSCLC based on various risk factors treated with PBT had similar outcomes to those with favorable-risk features.33 In the present study, overall survival and locoregional recurrence-free survival were comparable to the few published series of PBT as well. In particular, patients in our IMPT cohort were older, more often oxygen dependent, and more often receiving reirradiation compared with their IMRT counterparts; yet the clinical outcomes of the IMPT cohort were not inferior to the IMRT cohort. Our early results demonstrate that both IMPT and IMRT are acceptable treatment options for patients with LA-NSCLC. In addition, IMPT may be beneficial for patients with significant cardiopulmonary comorbidities or in the reirradiation setting and should be strongly considered for patients who have borderline performance status owing to these adverse factors.
Both IMPT and IMRT were well tolerated by our patients in the present study. Acute and subacute toxicities were limited and similar between the treatment cohorts. Only 9 (11%) of the 79 patients experienced a grade 3 or 4 toxicities in the subacute phase (3 months after RT). Grade 4 or higher toxicities were limited to dyspnea in a single patient with multiple cardiopulmonary comorbidities who developed a bronchopleural fistula which was multifactorial. As demonstrated by the treatment-related death in the IMRT cohort, careful consideration should be given for all patients with idiopathic pulmonary fibrosis. A recent randomized trial comparing passive scattering PBT versus IMRT by Liao et al demonstrated an overall 8.1% (10.5% PBT, 6.5% IMRT) grade ≥3 radiation pneumonitis rate.25 In their experience, passive scattering PBT exposed more lung tissue to doses ≥20 Gy (relative biologic effectiveness) than IMRT.25 However, large margins were required to treat these patients because passive scattering PBT relied on a 3-D conformal technique. As a result, passive scattering PBT did not improve radiation pneumonitis rates compared with IMRT. In contrast to passive scattering PBT, IMPT is intensity modulated, using a pencil-beam active-scanning delivery system representing a more advanced and conformal modality.34 IMPT delivers lower integral dose to healthy tissue than passive scattering PBT. Harnessing the advantages of IMPT can be challenging owing to tumor motion and interplay effects. However, our results have been consistent with multiple series demonstrating IMPT is safe and feasible.34, 35, 36 The present study demonstrated an overall subacute grade ≥3 radiation pneumonitis rate of 3.8% (6.1% IMPT, 2.2% IMRT).
RTOG 1308 is an ongoing trial randomizing patients with stage II to IIIB NSCLC to concurrent chemotherapy and either photons or protons (either passively scattering [3-D] or active-scanning [IMPT]).37 In addition, a prospective study directly comparing IMPT, the most advanced form of PBT, to IMRT is needed. This is most important in a high-risk population by age, cardiopulmonary comorbidities, and functional status.38
Reirradiation for recurrent NSCLC is an important treatment option; however, it is associated with significant toxicities. Despite being an ideal modality for reirradiation, there is limited data for PBT. Chao et al investigated reirradiation with both passive scattering and active scanning PBT in 57 patients from multiple institutions and demonstrated significant grade ≥3 (42%) toxicities and a direct correlation of toxicities to receipt of concurrent chemotherapy.39 Ho et al published an encouraging IMPT reirradiation experience of 27 patients and demonstrated late grade ≥3 pulmonary toxicity of 7%, no grade ≥3 esophagitis, and no grade 4 to 5 toxicities.36 Our study demonstrated no statistical difference in clinical outcomes or toxicities when comparing reirradiation patients directly to de novo patients. IMPT is a promising treatment option in the reirradiation setting, and we await further follow-up of our cohort to confirm these results.
Our study has several limitations; it was retrospective in nature and follow-up was not long. We did not adjust for tumoral mutational status or treatment with biologic and immunotherapy in our analyses and multivariate models, for which we will consider toward generation of future projects. In addition, there was an inherent bias in determining the radiation modality (IMPT vs IMRT) for a given patient on this study; as one would expect, older age, more severe cardiopulmonary comorbidities, and receipt of reirradiation were factors that may have encouraged the multidisciplinary team to choose IMPT as the treatment choice. Additionally, protons are approved for patients with Medicare who are older in general. We plan to analyze our prospectively collected patient-reported outcome data in the future.
Conclusion
Despite a far frailer IMPT cohort with older age, oxygen-dependence, and reirradiation status, our early experience suggests that IMPT and IMRT had similar outcomes in terms of survival and toxicities in locally advanced and recurrent NSCLC patients. IMPT is a safe and efficacious treatment that should be strongly considered for primary or recurrent LA-NSCLC patients who are older or with severe cardiopulmonary comorbidities. More clinical trials are needed as IMPT becomes a more commonly available treatment.
Footnotes
Sources of support: This work had no specific funding.
Disclosures: Dr Sio provides strategic and scientific recommendations as a member of the Advisory Board and speaker for Novocure, Inc, which is not in any way associated with the content or disease site as presented in this manuscript. Dr Schild writes for UpToDate on various subjects related to radiation oncology. All other authors have no financial or nonfinancial interests to be declared.
References
- 1.Alberg A.J., Brock M.V., Ford J.G., Samet J.M., Spivack S.D. Epidemiology of lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2013;143(5 Suppl):e1S–e29S. doi: 10.1378/chest.12-2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Molina J.R., Yang P., Cassivi S.D., Schild S.E., Adjei A.A. Non-small cell lung cancer: Epidemiology, risk factors, treatment, and survivorship. Mayo Clin Proc. 2008;83:584–594. doi: 10.4065/83.5.584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Govindan R., Bogart J., Vokes E.E. Locally advanced non-small cell lung cancer: The past, present, and future. J Thorac Oncol. 2008;3:917–928. doi: 10.1097/JTO.0b013e318180270b. [DOI] [PubMed] [Google Scholar]
- 4.Furuse K., Fukuoka M., Kawahara M. Phase III study of concurrent versus sequential thoracic radiotherapy in combination with mitomycin, vindesine, and cisplatin in unresectable stage III non-small-cell lung cancer. J Clin Oncol. 1999;17:2692–2699. doi: 10.1200/JCO.1999.17.9.2692. [DOI] [PubMed] [Google Scholar]
- 5.Curran W.J., Jr., Paulus R., Langer C.J. Sequential vs concurrent chemoradiation for stage III non-small cell lung cancer: Randomized phase III trial RTOG 9410. J Natl Cancer Inst. 2011;103:1452–1460. doi: 10.1093/jnci/djr325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aupérin A., Le Péchoux C., Rolland E. Meta-analysis of concomitant versus sequential radiochemotherapy in locally advanced non-small-cell lung cancer. J Clin Oncol. 2010;28:2181–2190. doi: 10.1200/JCO.2009.26.2543. [DOI] [PubMed] [Google Scholar]
- 7.Antonia S.J., Villegas A., Daniel D. Overall survival with Durvalumab after chemoradiotherapy in stage III NSCLC. N Engl J Med. 2018;379:2342–2350. doi: 10.1056/NEJMoa1809697. [DOI] [PubMed] [Google Scholar]
- 8.Kravchenko J., Berry M., Arbeev K. Cardiovascular comorbidities and survival of lung cancer patients: Medicare data based analysis. Lung Cancer. 2015;88:85–93. doi: 10.1016/j.lungcan.2015.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim Y.H., Ahn S.J., Kim Y.C. Predictive factors for survival and correlation to toxicity in advanced Stage III non-small cell lung cancer patients with concurrent chemoradiation. Jpn J Clin Oncol. 2016;46:144–151. doi: 10.1093/jjco/hyv174. [DOI] [PubMed] [Google Scholar]
- 10.Schild S.E., Stella P.J., Geyer S.M. The outcome of combined-modality therapy for stage III non-small-cell lung cancer in the elderly. J Clin Oncol. 2003;21:3201–3206. doi: 10.1200/JCO.2003.12.019. [DOI] [PubMed] [Google Scholar]
- 11.Stinchcombe T.E., Zhang Y., Vokes E.E. Pooled analysis of individual patient data on concurrent chemoradiotherapy for stage III non-small-cell lung cancer in elderly patients compared with younger patients who participated in US National Cancer Institute Cooperative group studies. J Clin Oncol. 2017;35:2885–2892. doi: 10.1200/JCO.2016.71.4758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schild S.E., Stella P.J., Geyer S.M. Phase III trial comparing chemotherapy plus once-daily or twice-daily radiotherapy in Stage III non-small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2002;54:370–378. doi: 10.1016/s0360-3016(02)02930-9. [DOI] [PubMed] [Google Scholar]
- 13.Bradley J.D., Paulus R., Komaki R. Standard-dose versus high-dose conformal radiotherapy with concurrent and consolidation carboplatin plus paclitaxel with or without cetuximab for patients with stage IIIA or IIIB non-small-cell lung cancer (RTOG 0617): A randomised, two-by-two factorial phase 3 study. Lancet Oncol. 2015;16:187–199. doi: 10.1016/S1470-2045(14)71207-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Palma D.A., Senan S., Tsujino K. Predicting radiation pneumonitis after chemoradiation therapy for lung cancer: An international individual patient data meta-analysis. Int J Radiat Oncol Biol Phys. 2013;85:444–450. doi: 10.1016/j.ijrobp.2012.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chun S.G., Hu C., Choy H. Impact of intensity-modulated radiation therapy technique for locally advanced non-small-cell lung cancer: A secondary analysis of the NRG Oncology RTOG 0617 randomized clinical trial. J Clin Oncol. 2017;35:56–62. doi: 10.1200/JCO.2016.69.1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McAvoy S., Ciura K., Wei C. Definitive reirradiation for locoregionally recurrent non-small cell lung cancer with proton beam therapy or intensity modulated radiation therapy: Predictors of high-grade toxicity and survival outcomes. Int J Radiat Oncol Biol Phys. 2014;90:819–827. doi: 10.1016/j.ijrobp.2014.07.030. [DOI] [PubMed] [Google Scholar]
- 17.Jeremić B., Videtic G.M. Chest reirradiation with external beam radiotherapy for locally recurrent non-small-cell lung cancer: A review. Int J Radiat Oncol Biol Phys. 2011;80:969–977. doi: 10.1016/j.ijrobp.2011.01.069. [DOI] [PubMed] [Google Scholar]
- 18.De Ruysscher D., Faivre-Finn C., Le Pechoux C., Peeters S., Belderbos J. High-dose re-irradiation following radical radiotherapy for non-small-cell lung cancer. Lancet Oncol. 2014;15:e620–e624. doi: 10.1016/S1470-2045(14)70345-6. [DOI] [PubMed] [Google Scholar]
- 19.Liu C., Sio T.T., Deng W. Small-spot intensity-modulated proton therapy and volumetric-modulated arc therapies for patients with locally advanced non-small-cell lung cancer: A dosimetric comparative study. J Appl Clin Med Phys. 2018;19:140–148. doi: 10.1002/acm2.12459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chang J.Y., Zhang X., Wang X. Significant reduction of normal tissue dose by proton radiotherapy compared with three-dimensional conformal or intensity-modulated radiation therapy in stage I or stage III non-small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2006;65:1087–1096. doi: 10.1016/j.ijrobp.2006.01.052. [DOI] [PubMed] [Google Scholar]
- 21.Higgins K.A., O'connell K., Liu Y. National cancer database analysis of proton versus photon radiation therapy in non-small cell lung cancer. Int J Radiat Oncol Biol Phys. 2017;97:128–137. doi: 10.1016/j.ijrobp.2016.10.001. [DOI] [PubMed] [Google Scholar]
- 22.Sejpal S., Komaki R., Tsao A. Early findings on toxicity of proton beam therapy with concurrent chemotherapy for nonsmall cell lung cancer. Cancer. 2011;117:3004–3013. doi: 10.1002/cncr.25848. [DOI] [PubMed] [Google Scholar]
- 23.Nguyen Q.N., Ly N.B., Komaki R. Long-term outcomes after proton therapy, with concurrent chemotherapy, for stage II-III inoperable non-small cell lung cancer. Radiother Oncol. 2015;115:367–372. doi: 10.1016/j.radonc.2015.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chang J.Y., Verma V., Li M. Proton beam radiotherapy and concurrent chemotherapy for unresectable stage III non-small cell lung cancer: Final results of a phase 2 study. JAMA Oncol. 2017;3:e172032. doi: 10.1001/jamaoncol.2017.2032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liao Z., Lee J.J., Komaki R. Bayesian adaptive randomization trial of passive scattering proton therapy and intensity-modulated photon radiotherapy for locally advanced non-small-cell lung cancer. J Clin Oncol. 2018;36:1813–1822. doi: 10.1200/JCO.2017.74.0720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu W., Schild S.E., Chang J.Y. Exploratory Study of 4D versus 3D Robust Optimization in Intensity Modulated Proton Therapy for Lung Cancer. Int J Radiat Oncol Biol Phys. 2016;95:523–533. doi: 10.1016/j.ijrobp.2015.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu C., Schild S.E., Chang J.Y. Impact of spot size and spacing on the quality of robustly-optimized intensity-modulated proton therapy plans for lung cancer. Int J Radiat Oncol Biol Phys. 2018;101:479–489. doi: 10.1016/j.ijrobp.2018.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yegya-raman N., Zou W., Nie K., Malhotra J., Jabbour S.K. Advanced radiation techniques for locally advanced non-small cell lung cancer: Intensity-modulated radiation therapy and proton therapy. J Thorac Dis. 2018;10(Suppl 21):S2474–S2491. doi: 10.21037/jtd.2018.07.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vyfhuis M.A.L., Onyeuku N., Diwanji T. Advances in proton therapy in lung cancer. Ther Adv Respir Dis. 2018;12 doi: 10.1177/1753466618783878. 1753466618783878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liao Z., Gandhi S.J., Lin S.H., Bradley J. Does proton therapy offer demonstrable clinical advantages for treating thoracic tumors? Semin Radiat Oncol. 2018;28:114–124. doi: 10.1016/j.semradonc.2017.11.002. [DOI] [PubMed] [Google Scholar]
- 31.Hoppe B.S., Henderson R., Pham D. A phase 2 trial of concurrent chemotherapy and proton therapy for stage III non-small cell lung cancer: Results and reflections following early closure of a single-institution study. Int J Radiat Oncol Biol Phys. 2016;95:517–522. doi: 10.1016/j.ijrobp.2015.11.004. [DOI] [PubMed] [Google Scholar]
- 32.Yang P., Xu T., Gomez D.R. Patterns of local-regional failure after intensity-modulated radiation therapy or passive scattering proton therapy with concurrent chemotherapy for non-small cell lung cancer. Int J Radiat Oncol Biol Phys. 2019;103:123–131. doi: 10.1016/j.ijrobp.2018.08.031. [DOI] [PubMed] [Google Scholar]
- 33.Zhu H.J., Nichols R.C., Henderson R.H. Impact of unfavorable factors on outcomes among inoperable stage II-IV nonsmall cell lung cancer patients treated with proton therapy. Acta Oncol. 2019;58:313–319. doi: 10.1080/0284186X.2018.1546060. [DOI] [PubMed] [Google Scholar]
- 34.Chang J.Y., Li H., Zhu X.R. Clinical implementation of intensity modulated proton therapy for thoracic malignancies. Int J Radiat Oncol Biol Phys. 2014;90:809–818. doi: 10.1016/j.ijrobp.2014.07.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li X., Kabolizadeh P., Yan D. Improve dosimetric outcome in stage III non-small-cell lung cancer treatment using spot-scanning proton arc (SPArc) therapy. Radiat Oncol. 2018;13:35. doi: 10.1186/s13014-018-0981-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ho J., Nguyen Q., Li H. Reirradiation of thoracic cancers with intensity modulated proton therapy. Prac Radiat Oncol. 2018;8:58–65. doi: 10.1016/j.prro.2017.07.002. [DOI] [PubMed] [Google Scholar]
- 37.Giaddui T., Chen W., Yu J. Establishing the feasibility of the dosimetric compliance criteria of RTOG 1308: Phase III randomized trial comparing overall survival after photon versus proton radiochemotherapy for inoperable stage II-IIIB NSCLC. Radiat Oncol. 2016;11:66. doi: 10.1186/s13014-016-0640-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Verma V., Rwigema J.M., Adeberg S., Simone C.B. Enrollment of elderly patients with locally advanced non-small cell lung cancer in multi-institutional trials of proton beam radiation therapy. Clin Lung Cancer. 2017;18:441–443. doi: 10.1016/j.cllc.2017.03.007. [DOI] [PubMed] [Google Scholar]
- 39.Chao H.H., Berman A.T., Simone C.B. Multi-institutional prospective study of reirradiation with proton beam radiotherapy for locoregionally recurrent non-small cell lung cancer. J Thorac Oncol. 2017;12:281–292. doi: 10.1016/j.jtho.2016.10.018. [DOI] [PubMed] [Google Scholar]