Abstract
Background
In order to identify suitable therapeutic targets for glioma anti-angiogenic therapy, the process of neovascularization mediated by circulating angiogenic cells (CACs) needs to be scrutinized.
Methods
In the present study, we compared the expression of neovascularization-related genes by 3 circulating CAC subsets (hematopoietic progenitor cells [HPCs], CD34+, and KDR+ cells; internal controls: peripheral blood mononuclear cells and circulating endothelial cells) of treatment-naïve patients with glioblastoma (GBM) to those of patients undergoing reactive neovascularization (myocardial infarction (MI). CACs from umbilical cord (representing developmental neovascularization) and healthy subjects served as controls. Fluorescent-activated cell sorting was used to isolate CACs, RT-PCR to determine the expression levels of a panel of 48 neovascularization-related genes, and Luminex assays to measure plasma levels of 21 CAC-related circulating molecules.
Results
We found essential differences in gene expression between GBM and MI CACs. GBM CACs had a higher expression of proangiogenic factors (especially, KITL, CXCL12, and JAG1), growth factor and chemotactic receptors (IGF1R, TGFBR2, CXCR4, and CCR2), adhesion receptor monomers (ITGA5 and ITGA6), and matricellular factor POSTN. In addition, we found major differences in the levels of neovascularization-related plasma factors. A strong positive correlation between plasma MMP9 levels and expression of CXCR4 in the CAC subset of HPCs was found in GBM patients.
Conclusions
Our findings indicate that CAC-mediated neovascularization in GBM is characterized by more efficient CAC homing to target tissue and a more potent proangiogenic response than in physiologic tissue repair in MI. Our findings can aid in selecting targets for therapeutic strategies acting against GBM-specific CACs.
Keywords: angiogenesis, circulating angiogenic cell, endothelial progenitor cell, glioma, hematopoietic progenitor cell, myocardial infarction, neovascularization
Key Points.
Glioblastoma CACs have a more potent homing and angiogenic capacity than controls.
CACs are programmed in the circulation by target tissue-specific requirements.
Unique CAC characteristics in different diseases translate to therapeutic targets.
Importance of the Study.
Prior literature on circulating angiogenic cells (CACs) in glioblastoma (GBM) uncovered their potent proangiogenic effects in vitro/vivo and their increased numbers in GBM patients. Our study is the first to show that GBM CACs are qualitatively different from non-neoplastic CACs (ie, in reactive [myocardial infarction], developmental [umbilical cord blood], and steady-state adult [healthy control] neovascularization). GBM CACs exhibit a gene expression profile compatible with increased tumor-homing capacity (higher expression of CXCR4, CCR2, ITGA5, and ITGA6) and a more potent proangiogenic potential (higher expression of KITL, CXCL12, JAG1, IGF1R, TGFBR2, and POSTN). Plasma levels of tumor-derived mobilization factor MMP9 correlate positively with both circulating hematopoietic progenitor cell (HPC) levels and HPC CXCR4 gene expression in GBM patients, illustrating that GBM tissue is capable of pre-programming CACs. GBM, though non-metastatic, should thus be considered a systemic disease requiring systemic treatment. Our results can be translated toward developing disease-specific therapies targeting CAC-induced neovascularization in GBM.
High-grade gliomas are among the most vascularized tumors and are characterized by an abundance of leaky vessels. Despite the high degree of vascularization, anti-angiogenic therapies have remained without the expected success.1 Anti-angiogenic drugs like bevacizumab interfere with Vascular Endothelial Growth Factor A (VEGFA) and the process of sprouting angiogenesis. However, the contribution of circulating cells engaged in the formation of blood vessels may be overlooked as a significant component of neovascularization in gliomas. This could partially explain the failing of anti-angiogenic therapies in glioma patients. Vasculogenesis is defined as de novo formation of blood vessels by endothelial progenitor cells (EPCs) that differentiate into endothelial cells and become part of the newly formed vessel wall.2 Although characteristic for embryogenesis, the process of vasculogenesis also contributes to neovascularization in adults.3 Whereas in embryogenesis differentiation into endothelial cells by EPCs is widespread, this process is limited in adulthood.4 In adulthood, circulating cells stimulate neovascularization by invading the target tissue and secreting proangiogenic factors that fuel angiogenesis4. Since these cells do not differentiate into endothelial cells, they do not fit the definition of EPC and are better termed “circulating angiogenic cells” (CACs). Various stages of CAC-mediated neovascularization exist. CACs are mobilized from the bone marrow by factors secreted by the target tissue and/or bone marrow microenvironment, or in an autocrine fashion by CACs themselves. In the bloodstream CACs migrate towards the target tissue through chemotaxis where they adhere to endothelial cells mediated by integrins and invade the tissue by expressing proteinases such as matrix metalloproteases (MMPs). Once in the target tissue CACs differentiate and start to secrete growth factors thus creating an environment permissive for angiogenesis.
In adulthood, neovascularization is stimulated on demand and is activated during revascularization after trauma or ischemia. In myocardial infarction (MI), a well-described and potent mobilization of CACs is induced early after the ischemic event.5 Other ischemic states, such as ischemic stroke, have been less extensively studied. The literature on CACs in ischemic stroke shows less consistent results regarding the mobilization of CACs, with some studies showing no increase6,7 or even a decrease of CACs.8 Since the CAC response to ischemic brain appears to be far less extensive than to ischemic myocardium,9 we chose to use MI patients rather than stroke patients as representing CAC-induced neovascularization in response to ischemia.
While in MI revascularization aids in recovery, new blood vessels in tumors are associated with propagation and contribute to the decease of the organism.10 In patients suffering from MI, CAC-based therapies have been implemented with promising results.11 In cancer, however, CAC-directed therapies have only been applied in animal studies where significant decreases in tumor sizes were reached.12 Little is known about functional differences in CAC trafficking and function in the contexts of acute ischemia, cancer, and development. A better understanding of CAC biology in these different situations is necessary to design therapies acting on CAC-related neovascularization in cancer.
Here we compared the expression in CAC subsets of genes involved in neovascularization of glioblastomas (GBMs) and MI. Umbilical cord blood (UCB) and blood from adult healthy controls (HC) served as references for embryonic/fetal and steady-state adult neovascularization, respectively. Genes and 21 circulating plasma factors were chosen based on their functional roles (mobilization, chemo-attraction, homing, and growth factors secretion).13 The expressional profiles of the respective CACs and the plasma factors of patients with GBM and MI were compared and correlated. The findings show profound differences between CAC-mediated neovascularization in GBM and MI patients.
Material and Methods
This study was approved by the Medical Ethics Committee of the Erasmus Medical Center, Rotterdam, The Netherlands (MEC-2011-313) and carried out in adherence to the Code of Good Conduct of the Federation of Medical Scientific Societies in the Netherlands (http://www.federa.org/codes-conduct). Informed consent was obtained from all subjects.
Blood Samples and Preparation: See Supplementary Materials and Methods.
Selection and FACS Sorting of CAC Subsets: See Supplementary Materials and Methods.
RNA Isolation and RT-PCR and Gene Expression Analysis: Quality Control: See Supplementary Materials and Methods.
RT-PCR Data Analysis: See Supplementary Materials and Methods.
Data Analysis: See Supplementary Materials and Methods.
Results
Hierarchical Cluster Analysis: Gene Expression Patterns of CAC Subsets From All Subjects
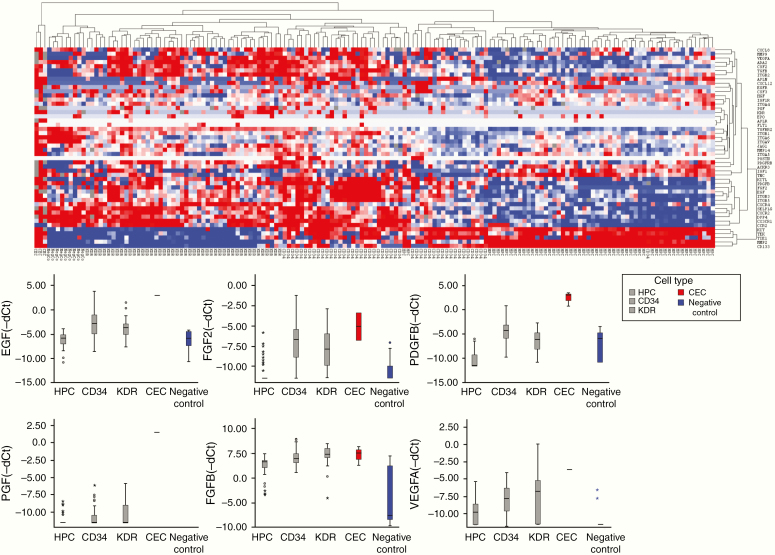
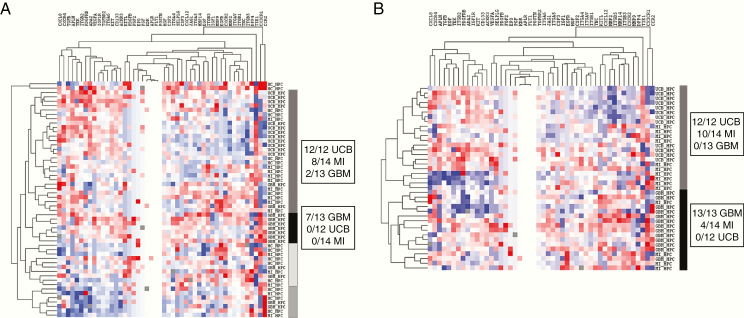
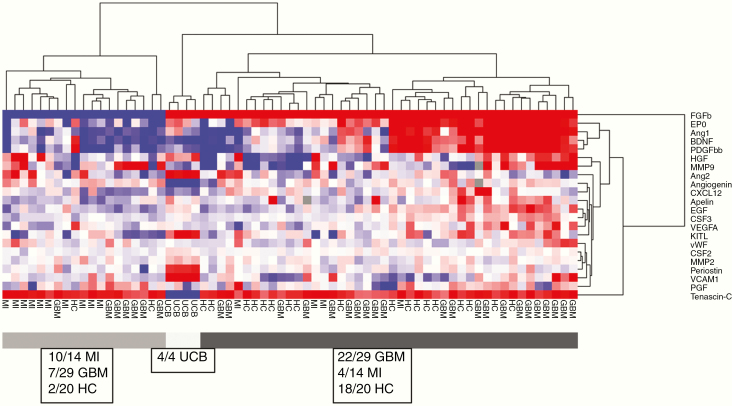
The expression patterns of the CAC subsets, negative control peripheral blood mononuclear cells (PBMCs), and circulating endothelial cells (CECs) in the various patient and control groups clustered according to the respective cell types (Figure 1). CECs expressed genes from almost all functional groups at a much higher level than the other CACs, except for chemotactic receptors, which were only expressed at a higher level in CECs compared to hematopoietic progenitor cells (HPCs). HPCs showed relatively low overall expression of neovascularization-related genes. Overall expression levels of the investigated genes were lower in HPCs than in KDR+ cells, CD34+ cells, and CECs. HPCs were most homogenous regarding gene expression, irrespective of the source of the blood samples. CD34+ cells clustered with HPCs for growth factor receptors and CD133 expression while they resembled KDR+ cells by their high expression of proangiogenic molecules and molecules operative in (de)adhesion and invasion. KDR+ cells clustered with negative control leukocytes for all functional groups, suggesting the closest kinship of all subsets investigated with negative control PMBCs. CACs from GBM patients expressed neovascularization-related genes at a higher level than those from MI patients or HC. Following unsupervised hierarchical cluster analysis on individual CAC subsets, we found that HPCs from UCB and MI clustered together, as opposed to GBM HPCs (Figure 2).
Figure 1.
Unsupervised hierarchical cluster analysis of gene expression in all samples and boxplots of expressional levels. Upper panel: Unsupervised hierarchical cluster analysis of gene expression in all samples (city block distance with complete linkage). Blue = low expression and red = high expression. Clustering is seen based on CAC type: CECs display the most conspicuous phenotype (high expression). CD34+ cells partially cluster with HPCs and partially with KDR+ cells. Negative control leukocytes cluster with KDR+ cells. The HPC cluster in general shows lower gene expression than the other CACs or CECs. Lower panel: Boxplots showing gene expression levels (−dCt) of proangiogenic factors in HPCs (n = 54), CD34+ cells (n = 47), KDR+ cells (n = 46), CECs (n = 3), and negative control PBMCs (n = 9). Proangiogenic factors overall are expressed highest in CECs and lowest in negative control PBMCs. In general, CD34+ and KDR+ cells express higher levels of proangiogenic factors than HPCs.
Figure 2.
Unsupervised hierarchical cluster analysis of gene expression in HPCs. (A) Unsupervised hierarchical cluster analysis (city block distance, complete linkage) of gene expression in HPCs (all samples). UCB and MI HPCs cluster together, while GBM HPCs are in a separate cluster. There is higher overall gene expression in the GBM HPCs cluster compared to the other clusters. (B) Unsupervised hierarchical cluster analysis of HPCs (city block distance, complete linkage) after removing HC samples from the analysis: clustering of UCB and MI HPCs is more obvious. There is higher gene expression in the GBM HPCs cluster compared to the other clusters.
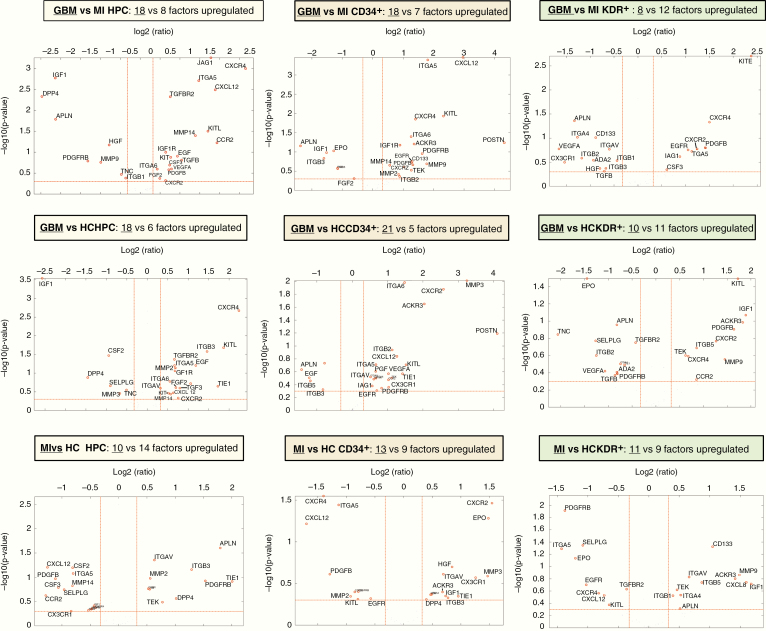
Differences in Expression of Individual Genes in CACs Between GBM and MI Patients
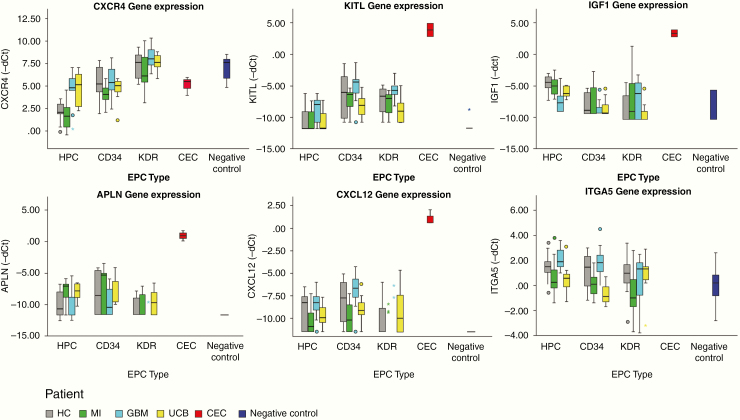
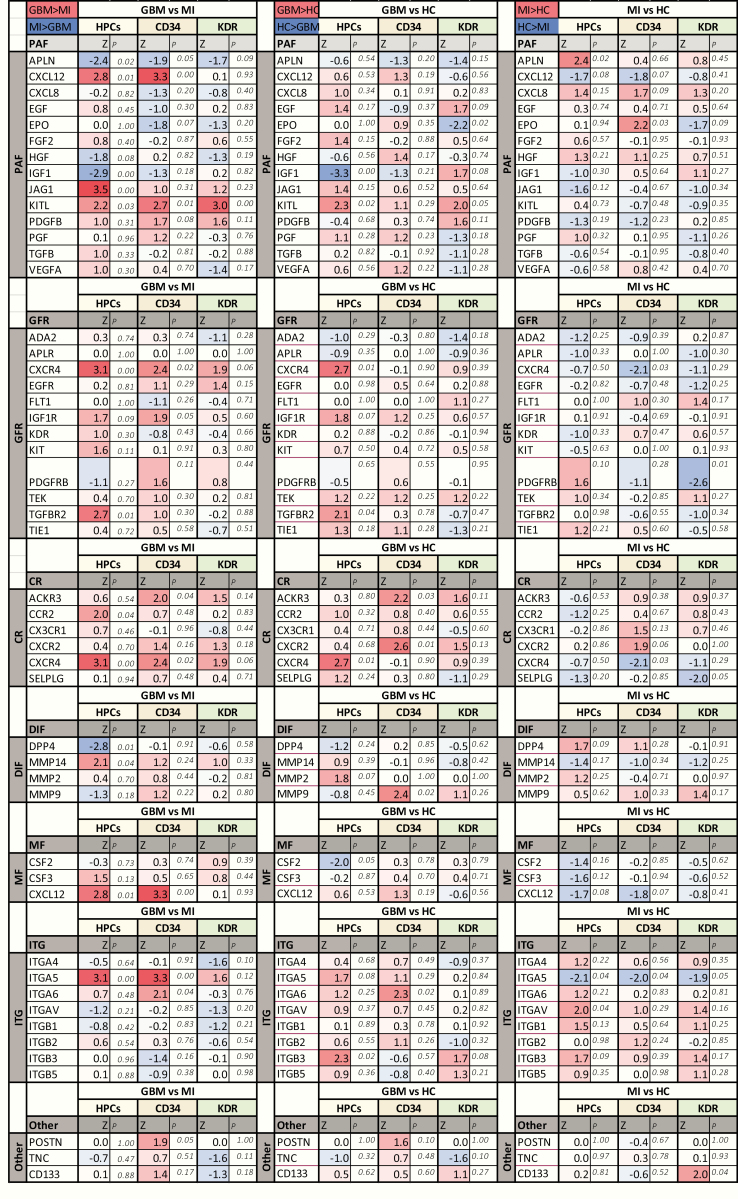
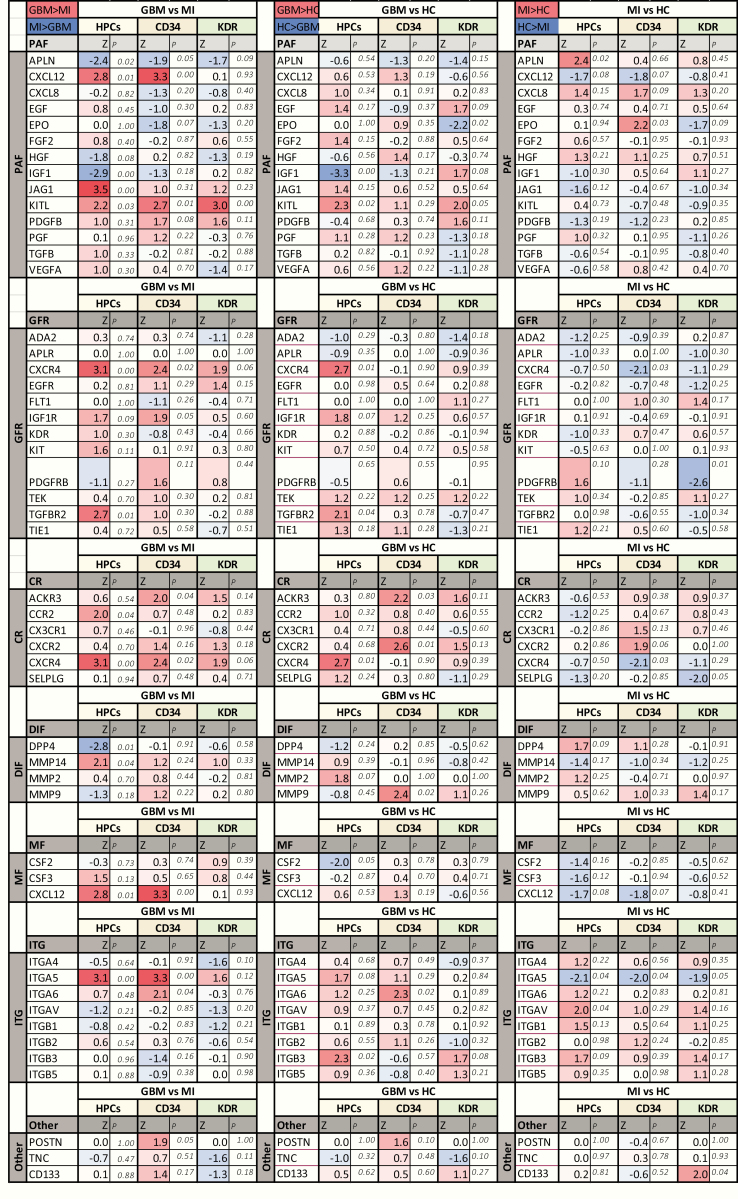
The genes that showed differential expression in CACs between the GBM and MI group represented all distinct functional groups (Figures 3–5). CXCR4 and KITL were overexpressed in all CAC subsets of GBM patients as compared to patients with MI. Reversely, IGF1 was underexpressed in GBM compared to MI. Higher RNA levels of APLN were detected in MI CACs as compared to GBM, while CXCL12 and ITGA5 transcript levels were lower in MI. The activity of some genes was consistently different for all CAC subtypes (eg, CXCR4 was overexpressed in GMB HPCs, CD34+, and KDR+ cells as compared to these cells in MI), while the differential activity of other genes appeared to be confined to specific CAC subtypes (eg, overexpression of JAG1 in GBM vs MI HPCs only, not in CD34+ or KDR+ CACs (Figures 3–5). Deviations from the reference HC expression levels (whether upregulated or downregulated) consistently followed the direction of UCB gene expression levels with the exception of KITL expression in GBM CACs (upregulated in GBM, downregulated in UCB compared to HC (Figures 4 and 5).
Figure 3.
Volcano plots of gene expression differences between patients and controls by CAC subset. Upper row (A–C): Volcano plots (−log10 P-value vs log2 fold change (FC) with the following cutoff values: FC > l1.25l, P < .5) of GBM versus MI CACs. More genes are overexpressed in GBM versus MI CACs. Overexpressed genes belong to all functional groups. Specifically, there is higher expression in GBM versus MI CACs (especially, HPCs and CD34+ cells) of growth factor receptors (GFRs), chemotactic receptors (CRs), and mobilization factors (MFs). There is higher expression in GBM versus MI HPCs of proangiogenic factors (PAFs). Z-scores and P-values of gene expression in GBM versus MI CACs are given in Figure 5. Middle row (D–F): Volcano plots (−log10 P-value vs log2 FC with the following cutoff values: FC > l1.25l, P < .5) of GBM versus HC CACs. A similar overall pattern of higher gene expression is seen as in the comparison of GBM versus MI CACs. Overexpressed genes belong to all functional groups. Higher expression in GBM versus HC CACs (especially, HPCs and CD34+ cells) of GFRs, CRs, MFs, adhesion factors (ITGs), PAFs. Lower row (G–I): Volcano plots (−log10 P-value vs log2 FC with the following cutoff values: FC > l1.25l, P < .5) of MI versus HC CACs. Overall gene expression is similar/lower in MI CACs versus HC CACs. Lower expression is seen in MI versus HC HPCs for PAFs, CRs, and MFs.
Figure 4.
Boxplots of gene expression levels (−dCt) of significantly differentially expressed genes between GBM and MI CACs. Boxplots showing gene expression levels (−dCt) of significantly differentially expressed genes between GBM and MI CACs (data shown for gene expression differences present in ≥2 CAC subsets). CXCR4 and KITL are overexpressed in GBM CACs compared to both MI and HC CACs. IGF1 is underexpressed in GBM CACs (HPCs and CD34+) compared to MI and HC CACs. ITGA5 is underexpressed in MI CACs compared to GBM and HC CACs. APLN is overexpressed in MI CACs compared to GBM and HC CACs. CXCL12 is underexpressed in MI CACs compared to GBM and HC CACs. Deviations from the reference HC expressions levels (whether upregulated or downregulated) follow the pattern of UCB CAC gene expression levels (except for the overexpression of KITL in GBM CACs). For exact P-values and Z-scores for each CAC subtype, see Figure 5.
Figure 5.
Differential gene expression between GBM, MI, and control groups. Z-scores and P-values of CAC subset gene expression (−dCt values) differences in patients and controls (Mann–Whitney U-test; SPSS version 25). Comparisons are made for each CAC subset included (HPCs, CD34+ cells, KDR+ cells) between patients (GBM, MI) and controls (HC, UCB). Genes are organized based on their function: PAFs, proangiogenic factors; GFRs, growth factor receptors; CRs, chemotactic receptors; DIFs, de-adhesion and invasion factors; MFs, mobilization factors; ITG, integrins (adhesion factors); Other, matricellular modulators of angiogenesis (POSTN/TNC) and the progenitor cell marker CD133.
Plasma Factors
In GBM patients the overall levels of all plasma factors were higher than those in MI patients and HC subjects. Unsupervised hierarchical cluster analysis of the concentrations of all plasma factors measured in all samples yielded 3 main clusters: one containing only UCB samples, one with the large majority of GBM and HC samples, and one with the large majority of MI samples (lower overall levels of plasma factors) (Figure 6). Spearman correlation analysis between plasma factor concentrations and gene expression in CACs revealed a strong positive correlation between plasma MMP9 levels and the expression of CXCR4 in HPCs in GBM patients (Spearman’s rho = 0.77; P < .01). In MI patients no correlation between HPC CXCR4 gene expression and plasma MMP9 levels was found (Supplementary Figure 3). When lowering the correlation threshold to at least 0.5, multiple significant correlations were detected between CAC gene expression and plasma factor levels (eg, a positive correlation between HPC CSF2 gene expression and plasma CXCL12 levels; positive correlation significant for both GBM and MI patients, not for HC).
Figure 6.
Plasma factors: unsupervised hierarchical cluster analysis and Z-scores. (A) Unsupervised hierarchical cluster analysis of plasma factors. GBM and MI samples are divided into different clusters. GBM patients have higher levels of many plasma factors. (B) Z-scores and P-values of plasma factors with significantly different levels in GBM, MI, and HC (P ≤ .05).
Discussion
In the present study, we investigated alterations in the expression of neovascularization-related genes in circulating CAC subsets between GBM and MI patients and sought correlations with circulating chemo-attractants and mobilization factors. Where in previous studies we observed that levels of circulating CACs differ in GBM patients as compared to HC and patients suffering from recent MIs,7,14 in the present study we explored the expression of 48 neovascularization-related genes in 3 CAC subsets in these groups. We found major differences in expressional profiles. There was close similarity between the gene expression patterns of HPCs in MI and UCB, indicative of reactivation of embryonal/fetal mechanisms for CAC-mediated neovascularization following acute myocardial ischemia. In circulating CACs from GBM (where neovascularization is disordered and haphazard) this coordinated CAC gene expression program was absent. We also discovered significant variations in the concentrations of 21 neovascularization-related plasma factors between GBM and MI patients, reflecting considerable differences in the “microenvironment” of the peripheral circulation, in which circulating CACs reside. Furthermore, we found strong correlations between the levels of specific plasma factors and gene expression levels in CACs. Altogether, these findings suggest that the difference in “blood microenvironment” as a result of MI or neoplastic growth drives alterations in gene expression in circulating CACs.
HPCs are capable of trafficking back and forth between the bone marrow, peripheral blood, (extra)-medullary tissues, and the lymphatic system.15 We know from the literature that HPCs mobilized to peripheral blood have different gene expression profiles than bone marrow (BM)-resident HPCs.16 Hypothetically, residing in target tissues will alter HPC (and other CAC) gene expression profiles dependent on target tissue/lesion-specific microenvironments. Hence, another explanation for our findings of altered gene expression patterns in CACs between GBM and MI patients is the reentrance of CACs that were reprogrammed in such target tissues into the bloodstream. Differences in the trafficking speed of CACs between the bone marrow, peripheral blood, and target tissues can also contribute to changes in particular gene expression patterns16 and could be another factor contributing to our findings. The trafficking speed is dependent on various circumstances, such as levels of mobilization factors in the circulation and sympathetic innervation of BM.17 The latter could be altered in the presence of malignant glioma. Various combinations of cues like adhesion/chemotactic receptors, not single molecules themselves, drive the attraction and retention of HPCs to specific niches in the bone marrow.18,19 It is likely that similar cue patterns govern the attraction and retention of CACs to specific target tissues. We found that these cues differ in the context of GBM and MI, pointing to disease-driven alterations in gene expression in circulating CACs. The CAC gene expression profile in GBM patients suggests that they have a more potent capacity to home to GBM tissue and are capable of a stronger proangiogenic response than CACs in MI. Overall, the influence of GBM tumor tissue on circulating CAC biology justifies the notion that GBM should be considered as a systemic disease, rather than a disease which is limited to the brain.
The expression level of CXCR4 in GBM CACs was similar to that in UCB CACs, but significantly higher than in MI CACs. CXCR4 is a chemokine receptor expressed on the surface of leukocytes and HPCs.20 CXCR4 binds to its ligand CXCL12, which acts as a mobilization factor and chemoattractant of CXCR4+ cells, including HPCs. Because CXCL12 is highly expressed in GBM tumor cells, endothelial cells, neurons, and white matter we included this protein in our panel of plasma factors.21–24 We found decreased plasma CXCL12 levels in both GBM and MI patients. The lower CXCL12 levels in MI patients are in line with the existing literature,25 while in glioma patients elevated, not reduced, plasma levels of CXCL12 have been reported.26 A technical explanation for the reported elevated levels could be the release of α-granule factors including CXCL12 into plasma following blood sample cooling.26,27 In our study plasma values represent the free CXCL12 fraction, not the platelet α-granule stored fraction. While high free plasma CXCL12 mobilizes CACs from the bone marrow, homing of CXCR4+ cells to target tissues is less efficient due to the lower target tissue-to-plasma CXCL12 ratio.28,29 Reversely, low plasma CXCL12 levels allow for more efficient homing of CXCR4+ cells to CXCL12-expressing target tissues due to a high target tissue-to-plasma CXCL12 ratio.30 The low plasma level of CXCL12 in GBM patients therefore facilitates homing of CXCR4+ cells to CXCL12-expressing GBM tissue.30,31 The present finding of increased expression of CXCR4 in UCB HPCs was previously reported in the literature,32 but increased CXCR4 expression in GBM HPCs (and other CACs) was not described earlier. Higher expression of CXCR4 in cultured CACs increases migration triggered by CXCL12 and enhances their capacity to exit blood vessels and improve endothelial recovery.33 In MI strategies to increase the expression of CXCR4 by circulating progenitor cells lead to improved homing to ischemic myocardium resulting in restoration of the blood flow and a reduction of cardiac damage following the infarction.24 MMP9 not only induces mobilization of HPCs by cleaving the CXCL12–CXCR4 interaction,34 but also increases the expression of CXCR4 by bone marrow progenitor cells.35,36 The increased plasma levels of MMP9 in GBM patients found in the present study corroborate the literature.37 The elevated levels of tumor-derived MMP9 could cause upregulation of CXCR4 in CACs of GBM patients. Furthermore, the reduced expression of DPP4 in GBM HPCs is also associated with a more efficient homing of HPCs to CXCL12-expressing target tissue.38 It is therefore likely that the elevated expression of CXCR4 and the reduced expression of DPP4 by GBM CACs, combined with the high GBM tissue-to-plasma CXCL12 gradient, translate into a highly efficient homing process of CXCR4+ CACs to GBM tumor. Interference with the MMP9/DPP4/CXCR4/CXCL12 axis in CACs in GBM patients seems a very promising therapeutic option for targeting CAC-mediated neovascularization.
In GBM CACs, gene expression of KITL was significantly higher than in MI and HC. KIT was expressed higher in GBM than in MI HPCs. KITL is a cytokine that binds to the KIT receptor; the KIT/KITL receptor/ligand pair is important for hematopoiesis and for the mobilization, chemotaxis/homing, and maintenance of HPCs,39,40 as well as for angiogenesis.41–43 The KIT/KITL axis is also essential for neovascularization in glial tumors.41 In GBM tissue, KITL is not only produced by glial tumor cells, but also by neurons.41 Silencing of KITL in glioma cells leads to a decrease in angiogenesis and tumor growth and improved survival.41 The KIT receptor is widely expressed in GBM endothelial cells and in tumor cells present around foci of necrosis.44 KITL exists in a soluble (sKITL) and membrane bound (mKITL) form.45 sKITL results from proteolytic cleavage of mKITL.42 Transmembrane KITL is formed by alternative mRNA splicing. The proteolytic cleavage of mKITL to sKITL by MMPs (in particular MMP9) is crucial for the mobilization of HPCs from the bone marrow in a similar fashion as for CXCR4/CXCL12.46,47 Indeed, we previously found a strong correlation between plasma MMP9 levels and circulating levels of HPCs in GBM patients.14 In the present study, the primer set used to determine KITL mRNA levels did not distinguish between the soluble and transmembrane forms. Hence, we do not yet know if the increased KITL gene expression translates to higher levels of sKITL, mKITL, or both in GBM CACs. Importantly, mKITL can act as a chemotactic membrane bound ligand to KIT+ cells in the target tissue,48 mediating the homing of mKITL+ cells to KIT+ target tissue. Reversely, KIT+ circulating progenitor cells home to KITL+ target tissue.49 Hence, the high KITL expression by GBM CACs, and the high KIT expression by GBM HPCs, is expected to facilitate homing to KIT+/KITL+ GBM tissue and stimulate tumor angiogenesis. The role of KIT/KITL in GBM CACs therefore deserves further investigations in the search for targets for CACs-induced neovascularization in GBM.
The functional meaning of our findings should be explored further using in vitro and ex vivo experimental systems, in animal models and finally in clinical trials on humans. FACS or immunomagnetic bead-isolated CACs could be used in chemotaxis/invasion assays (transwell) to determine the potential of GBM versus MI/HC CACs to migrate along gradients of chemoattractants (eg, CXCL12, CCL2, sKITL, sKIT, and sVCAM1) and/or to GBM cells. Silencing of CXCR4, KIT/KITL, and ITGA5/ITGA4 in CACs or the addition of CXCR4 blockers (such as AMD3100) or KITL/KIT/Intα5β1/Intα4β1 inhibitors could be used to validate the importance of these factors in the chemoattraction/homing response. Additionally, CACs could be treated with MMP9 to determine its effect on CAC CXCR4 expression and chemotaxis. The angiogenic function of GBM CACs in GBM could be confirmed using 3D angiogenesis assays.50 Labeled CACs (GBM vs MI/HC) could be injected into the circulation and tumor tissue of a GBM xenograft orthotopic mouse model to determine their tumor-homing capacity and their effect on tumor neovascularization and growth. CACs could be isolated from GBM tissue after having homed to tumor, and their expression profile compared to the original CACs to determine the effect of the GBM microenvironment on CAC gene expression. Inhibition of homing molecules like CXCR4, KITL/KIT, and Intα5β1/Intα4β1 prior to peripheral administration of CACs would validate the function of these molecules in vivo. Finally, clinical trials can be developed investigating the effect of blocking the mobilization and/or tumor homing of CACs on GBM neovascularization and growth (eg, by blocking circulating MMP9 or VCAM1, both elevated in GBM patient plasma and correlating positively with levels of HPCs and KDR+ cells, respectively14). Lowering the levels of plasma MMP9 would reduce CAC CXCR4 expression35,36 and diminish their homing capacity to tumor CXCL12. Similarly, blockage of CXCR4 using, eg, AMD3100 could abrogate the homing potential of CACs.51 Since AMD3100 also mobilizes CACs from the bone marrow, alternative homing mechanisms than the CXCR4/CXCL12 axis may need to be targeted simultaneously to prevent CACs from reaching GBM tissue using alternative routes (eg, KIT/KITL, Intα4β1/VCAM1).
Our results can eventually be translated toward developing disease-specific therapies targeting CAC-induced neovascularization. Crucial to the development of these targeted therapies is maintaining the balance between effective anti-angiogenic therapy and preservation of the necessary regenerative capacities of the organism.
Funding
The funding for this project was provided by the Department of Pathology of the Erasmus Medical Center.
Supplementary Material
Acknowledgments
Dr A. M. Sieuwerts† for her expertise and help with setting up and validating the rare-cell RT-PCR experiments; Dr R. J. M. van Geuns, Department of Cardiology for collection of the MI patients’ blood samples; Prof. Dr R. Fodde for granting access to the FACS facility; Mr F. van der Panne for his assistance with creating the figures.
Conflict of interest statement. None of the authors have any conflicts of interest to disclose.
Authorship Statement K.H., J.M.K., D.A.M., and A.S. contributed to formation of hypotheses, experimental design, implementation and interpretation of the data, and writing the manuscript. S.S. and P.J.v.d.S. contributed to data analysis. W.D. contributed to experimental design, implementation, and data interpretation regarding Luminex assays.
References
- 1. Xiao Q, Yang S, Ding G, Luo M. Anti-vascular endothelial growth factor in glioblastoma: a systematic review and meta-analysis. Neurol Sci. 2018;39(12):2021–2031. [DOI] [PubMed] [Google Scholar]
- 2. Carmeliet P. Mechanisms of angiogenesis and arteriogenesis. Nat Med. 2000;6(4):389–395. [DOI] [PubMed] [Google Scholar]
- 3. Drake CJ. Embryonic and adult vasculogenesis. Birth Defects Res C Embryo Today. 2003;69(1):73–82. [DOI] [PubMed] [Google Scholar]
- 4. Fang S, Salven P. Stem cells in tumor angiogenesis. J Mol Cell Cardiol. 2011;50(2):290–295. [DOI] [PubMed] [Google Scholar]
- 5. Wojakowski W, Landmesser U, Bachowski R, Jadczyk T, Tendera M. Mobilization of stem and progenitor cells in cardiovascular diseases. Leukemia. 2012;26(1):23–33. [DOI] [PubMed] [Google Scholar]
- 6. Aragona CO, Imbalzano E, Mamone F, et al. . Endothelial progenitor cells for diagnosis and prognosis in cardiovascular disease [published online ahead of print December 29, 2015]. Stem Cells Int. 2016;2016:8043792. doi: 10.1155/2016/8043792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zheng PP, Hop WC, Luider TM, Sillevis Smitt PA, Kros JM. Increased levels of circulating endothelial progenitor cells and circulating endothelial nitric oxide synthase in patients with gliomas. Ann Neurol. 2007;62(1):40–48. [DOI] [PubMed] [Google Scholar]
- 8. Lau KK, Chan YH, Yiu KH, et al. . Burden of carotid atherosclerosis in patients with stroke: relationships with circulating endothelial progenitor cells and hypertension. J Hum Hypertens. 2007;21(6): 445–451. [DOI] [PubMed] [Google Scholar]
- 9. Regueiro A, Cuadrado-Godia E, Bueno-Betí C, et al. . Mobilization of endothelial progenitor cells in acute cardiovascular events in the PROCELL study: time-course after acute myocardial infarction and stroke. [published online ahead of print January 22, 2015] J Mol Cell Cardiol. 2015;80:146–155. doi: 10.1016/j.yjmcc.2015.01.005. [DOI] [PubMed] [Google Scholar]
- 10. Moccia F, Zuccolo E, Poletto V, et al. . Endothelial progenitor cells support tumour growth and metastatisation: implications for the resistance to anti-angiogenic therapy. Tumour Biol. 2015;36(9):6603–6614. [DOI] [PubMed] [Google Scholar]
- 11. Jeevanantham V, Butler M, Saad A, Abdel-Latif A, Zuba-Surma EK, Dawn B. Adult bone marrow cell therapy improves survival and induces long-term improvement in cardiac parameters: a systematic review and meta-analysis. Circulation. 2012;126(5):551–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lyden D, Hattori K, Dias S, et al. . Impaired recruitment of bone-marrow-derived endothelial and hematopoietic precursor cells blocks tumor angiogenesis and growth. Nat Med. 2001;7(11):1194–1201. [DOI] [PubMed] [Google Scholar]
- 13. Caiado F, Dias S. Endothelial progenitor cells and integrins: adhesive needs. Fibrogenesis Tissue Repair. 2012;5:4. doi: 10.1186/1755-1536-5-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Huizer K, Sacchetti A, Dik WA, Mustafa DA, Kros JM. Circulating proangiogenic cells and proteins in patients with glioma and acute myocardial infarction: differences in neovascularization between neoplasia and tissue regeneration. J Oncol. 2019;2019:3560830. doi: 10.1155/2019/3560830. eCollection 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mazo IB, Massberg S, von Andrian UH. Hematopoietic stem and progenitor cell trafficking. Trends Immunol. 2011;32(10):493–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Steidl U, Kronenwett R, Haas R. Differential gene expression underlying the functional distinctions of primary human CD34+ hematopoietic stem and progenitor cells from peripheral blood and bone marrow. Ann N Y Acad Sci. 2003;996:89–100. PMID: 12799287. doi: 10.1111/j.1749-6632.2003.tb03237.x. [DOI] [PubMed] [Google Scholar]
- 17. Larsson J, Scadden D. Nervous activity in a stem cell niche. Cell. 2006;124(2):253–255. [DOI] [PubMed] [Google Scholar]
- 18. Ugarte F, Forsberg EC. Haematopoietic stem cell niches: new insights inspire new questions. EMBO J. 2013;32(19):2535–2547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Forsberg EC, Smith-Berdan S. Parsing the niche code: the molecular mechanisms governing hematopoietic stem cell adhesion and differentiation. Haematologica. 2009;94(11):1477–1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Dar A, Kollet O, Lapidot T. Mutual, reciprocal SDF-1/CXCR4 interactions between hematopoietic and bone marrow stromal cells regulate human stem cell migration and development in NOD/SCID chimeric mice. Exp Hematol. 2006;34(8):967–975. [DOI] [PubMed] [Google Scholar]
- 21. Salmaggi A, Gelati M, Pollo B, et al. . CXCL12 in malignant glial tumors: a possible role in angiogenesis and cross-talk between endothelial and tumoral cells. J Neurooncol. 2004;67(3):305–317. [DOI] [PubMed] [Google Scholar]
- 22. Zagzag D, Esencay M, Mendez O, et al. . Hypoxia- and vascular endothelial growth factor-induced stromal cell-derived factor-1alpha/CXCR4 expression in glioblastomas: one plausible explanation of Scherer’s structures. Am J Pathol. 2008;173(2):545–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Jiang Z, Zhou W, Guan S, Wang J, Liang Y. Contribution of SDF-1α/CXCR4 signaling to brain development and glioma progression. Neurosignals. 2013;21(3–4):240–258. [DOI] [PubMed] [Google Scholar]
- 24. Takahashi M. Role of the SDF-1/CXCR4 system in myocardial infarction. Circ J. 2010;74(3):418–423. [DOI] [PubMed] [Google Scholar]
- 25. Stellos K, Ruf M, Sopova K, et al. . Plasma levels of stromal cell-derived factor-1 in patients with coronary artery disease: effect of clinical presentation and cardiovascular risk factors. Atherosclerosis. 2011;219(2):913–916. [DOI] [PubMed] [Google Scholar]
- 26. Greenfield JP, Jin DK, Young LM, et al. . Surrogate markers predict angiogenic potential and survival in patients with glioblastoma multiforme. Neurosurgery. 2009;64(5):819–826; discussion 826–817. [DOI] [PubMed] [Google Scholar]
- 27. Chatterjee M, Gawaz M. Platelet-derived CXCL12 (SDF-1α): basic mechanisms and clinical implications. J Thromb Haemost. 2013;11(11):1954–1967. [DOI] [PubMed] [Google Scholar]
- 28. Döring Y, Pawig L, Weber C, Noels H. The CXCL12/CXCR4 chemokine ligand/receptor axis in cardiovascular disease. Front Physiol. 2014;5:212. doi: 10.3389/fphys.2014.00212. eCollection 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Berahovich RD, Zabel BA, Lewén S, et al. . Endothelial expression of CXCR7 and the regulation of systemic CXCL12 levels. Immunology. 2014;141(1):111–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Madlambayan GJ, Butler JM, Hosaka K, et al. . Bone marrow stem and progenitor cell contribution to neovasculogenesis is dependent on model system with SDF-1 as a permissive trigger. Blood. 2009;114(19):4310–4319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Stellos K, Gawaz M. Platelets and stromal cell-derived factor-1 in progenitor cell recruitment. Semin Thromb Hemost. 2007;33(2):159–164. [DOI] [PubMed] [Google Scholar]
- 32. Yong K, Fahey A, Reeve L, et al. . Cord blood progenitor cells have greater transendothelial migratory activity and increased responses to SDF-1 and MIP-3beta compared with mobilized adult progenitor cells. Br J Haematol. 1999;107(2):441–449. [DOI] [PubMed] [Google Scholar]
- 33. Chen L, Wu F, Xia WH, et al. . CXCR4 gene transfer contributes to in vivo reendothelialization capacity of endothelial progenitor cells. Cardiovasc Res. 2010;88(3):462–470. [DOI] [PubMed] [Google Scholar]
- 34. Klein G, Schmal O, Aicher WK. Matrix metalloproteinases in stem cell mobilization. [published online ahead of print January 21, 2015] Matrix Biol. 2015;44–46:175–183. doi: 10.1016/j.matbio.2015.01.011. [DOI] [PubMed] [Google Scholar]
- 35. Kawai K, Xue F, Takahara T, et al. . Matrix metalloproteinase-9 contributes to the mobilization of bone marrow cells in the injured liver. Cell Transplant. 2012;21(2–3):453–464. [DOI] [PubMed] [Google Scholar]
- 36. Lapid K, Glait-Santar C, Gur-Cohen S, Canaani J, Kollet O, Lapidot T. Egress and mobilization of hematopoietic stem and progenitor cells: a dynamic multi-facet process. StemBook. Cambridge, MA: Harvard Stem Cell Institute;2008. [PubMed] [Google Scholar]
- 37. Lin Y, Wang JF, Gao GZ, Zhang GZ, Wang FL, Wang YJ. Plasma levels of tissue inhibitor of matrix metalloproteinase-1 correlate with diagnosis and prognosis of glioma patients. Chin Med J (Engl). 2013;126(22):4295–4300. [PubMed] [Google Scholar]
- 38. Christopherson KW 2nd, Hangoc G, Mantel CR, Broxmeyer HE. Modulation of hematopoietic stem cell homing and engraftment by CD26. Science. 2004;305(5686):1000–1003. [DOI] [PubMed] [Google Scholar]
- 39. Dutt P, Wang JF, Groopman JE. Stromal cell-derived factor-1 alpha and stem cell factor/kit ligand share signaling pathways in hemopoietic progenitors: a potential mechanism for cooperative induction of chemotaxis. J Immunol. 1998;161(7):3652–3658. [PubMed] [Google Scholar]
- 40. Fazel SS, Chen L, Angoulvant D, et al. . Activation of c-kit is necessary for mobilization of reparative bone marrow progenitor cells in response to cardiac injury. FASEB J. 2008;22(3):930–940. [DOI] [PubMed] [Google Scholar]
- 41. Sun L, Hui AM, Su Q, et al. . Neuronal and glioma-derived stem cell factor induces angiogenesis within the brain. Cancer Cell. 2006;9(4):287–300. [DOI] [PubMed] [Google Scholar]
- 42. Lennartsson J, Rönnstrand L. Stem cell factor receptor/c-Kit: from basic science to clinical implications. Physiol Rev. 2012;92(4):1619–1649. [DOI] [PubMed] [Google Scholar]
- 43. Kim KL, Meng Y, Kim JY, Baek EJ, Suh W. Direct and differential effects of stem cell factor on the neovascularization activity of endothelial progenitor cells. Cardiovasc Res. 2011;92(1):132–140. [DOI] [PubMed] [Google Scholar]
- 44. Sihto H, Tynninen O, Bützow R, Saarialho-Kere U, Joensuu H. Endothelial cell KIT expression in human tumours. J Pathol. 2007;211(4):481–488. [DOI] [PubMed] [Google Scholar]
- 45. Smith MA, Court EL, Smith JG. Stem cell factor: laboratory and clinical aspects. Blood Rev. 2001;15(4):191–197. [DOI] [PubMed] [Google Scholar]
- 46. Grünwald B, Vandooren J, Gerg M, et al. . Systemic ablation of MMP-9 triggers invasive growth and metastasis of pancreatic cancer via deregulation of IL6 expression in the bone marrow. Mol Cancer Res. 2016;14(11): 1147–1158. [DOI] [PubMed] [Google Scholar]
- 47. Heissig B, Hattori K, Dias S, et al. . Recruitment of stem and progenitor cells from the bone marrow niche requires MMP-9 mediated release of kit-ligand. Cell. 2002;109(5):625–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Wang Y, Luther K. Genetically manipulated progenitor/stem cells restore function to the infarcted heart via the SDF-1α/CXCR4 signaling pathway. Prog Mol Biol Transl Sci. 2012;111:265–284. doi: 10.1016/B978-0-12-398459-3.00012-5. [DOI] [PubMed] [Google Scholar]
- 49. Lutz M, Rosenberg M, Kiessling F, et al. . Local injection of stem cell factor (SCF) improves myocardial homing of systemically delivered c-kit + bone marrow-derived stem cells. Cardiovasc Res. 2008;77(1): 143–150. [DOI] [PubMed] [Google Scholar]
- 50. Zhu C, Chrifi I, Mustafa D, et al. . CECR1-mediated cross talk between macrophages and vascular mural cells promotes neovascularization in malignant glioma. Oncogene. 2017;36(38): 5356–5368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Gagner JP, Sarfraz Y, Ortenzi V, et al. . Multifaceted C-X-C chemokine receptor 4 (CXCR4) inhibition interferes with anti-vascular endothelial growth factor therapy-induced glioma dissemination. Am J Pathol. 2017;187(9):2080–2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.