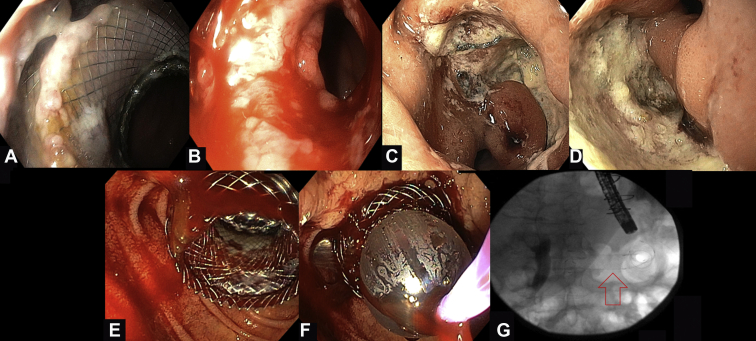
Figure 1.
Intraprocedural and postprocedural lumen-apposing metal stent (LAMS)-related adverse events during EUS–directed transgastric ERCP. A, Endoscopic view from the proximal end of the jejunogastrostomy 1 month after the procedure revealing the embedded LAMS. B, Endoscopic view right after the removal of the LAMS. Minor bleeding can be noted. C, D, Endoscopic images 9 months after the procedure. Two large, nonbleeding ulcers at the surgical gastrojejunostomy site. E, Moderate bleeding that occurred after dilation of the stent. F, Reinsertion of the balloon catheter through the stent and application of tamponade with 18-mm inflated balloon for 1.5 minutes. G, Fluoroscopic image showing the fully covered esophageal stent across the LAMS.