Table 8.
IC50 and Ki values of phosphamides hydroxamic acid-based inhibitors.
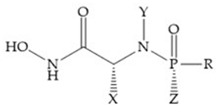
|
IC50 (X = H; Y = (CH2)2C6H5; Z = Me; R = Ph): MMP-1 = 252 nM; MMP-3 = 700 nM IC50 (X = H; Y = (CH2)2C6H5; Z = Ph; R = Ph): MMP-1 = 854 nM; MMP-3 = 1.75 μM IC50 (X = Me; Y = CH2C6H5; Z = Me; R = Ph): MMP-1 = 120 nM; MMP-3 = 67.9 nM IC50 (X = Me; Y = CH2C6H5; Z = Et; R = Ph): MMP-1 = 608 nM; MMP-3 = 700 nM IC50 (X = Me; Y = CH2C6H5; Z = Ph; R = Ph): MMP-1 = 6.79 μM; MMP-3 = 10.3 μM IC50 (X = CH2i-Pr; Y = CH2C6H5; Z = Me; R = Ph): MMP-1 = 20.5 nM; MMP-3 = 24.4 nM IC50 (X = CH2i-Pr; Y = CH2C6H5; Z = Me; R = Me): MMP-1 = 518 nM; MMP-3 = 1.04 μM |
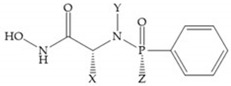
|
IC50 (X = CH2i-Pr; Y = H; Z = CH3): R isómer, MMP-1 = 2.51 μM; MMP-3 = 2.55 μM S isomer: MMP-1 > 100 μM; MMP-3 = 130.5 μM IC50 (X = CH2i-Pr; Y = CH2C6H5; Z = CH3): R isomer, MMP-1 = 20.5 nM; MMP-3 = 24.4 nM S isomer, MMP-1 = 7.12 μM; MMP-3 = 9.17 μM IC50 (X = CH3; Y = CH2C6H5; Z = C2H5): R isomer, MMP-1 = 608 nM; MMP-3 = 700 nM S isomer, MMP-1 = 33.3 μM; MMP-3 = 49.3 μM |
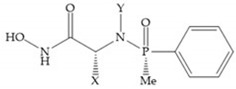
|
IC50 (X = H; Y = (CH2)2C6H5): MMP-1 = 525 nM; MMP-3 = 700 nM IC50 (X = CH3; Y = CH2C6H5): MMP-1 = 120 nM; MMP-3 = 67.9 nM IC50 (X = CH3; Y = n-C6H13): MMP-1 = 1.29 μM; MMP-3 = 1.6 μM IC50 (X = CH2i-Pr; Y = H): MMP-1 = 2.51 μM; MMP-3 = 2.55 μM IC50 (X = CH2i-Pr; Y = CH2C6H5): MMP-1 = 20.5 nM; MMP-2 = 13.3 nM; MMP-3 = 24.4 nM; MMP-7 = 886 nM; MMP-8 = 5.3 nM; MMP-9 = 20.6 nM; MMP-13 = 7.4 nM |
