Abstract
Given the high prevalence (1 in 40) of BRCA1 and BRCA2 mutations among Ashkenazi Jews, population-based BRCA genetic testing in this ethnic subgroup may detect more mutation carriers. We conducted a cross-sectional survey among Orthodox Jewish women in New York City to assess breast cancer risk, genetic testing knowledge, self-efficacy, perceived breast cancer risk and worry, religious and cultural factors affecting medical decision-making. We used descriptive statistics and multivariable logistic regression models to identify predictors of genetic testing intention/uptake. Among evaluable respondents (n = 243, 53% response rate), median age was 25 and nearly half (43%) had a family history of breast cancer. Only 49% of the women had adequate genetic testing knowledge and 46% had accurate breast cancer risk perceptions. Five percent had already undergone BRCA genetic testing, 20% stated that they probably/definitely will get tested, 28% stated that they probably/definitely will not get tested, and 46% had not thought about it. High decision self-efficacy, adequate genetic testing knowledge, higher breast cancer risk, and overestimation of risk were associated with genetic testing intention/uptake. Decision support tools that improve knowledge and self-efficacy about genetic testing may facilitate population-based BRCA testing among Orthodox Jews.
Keywords: Ashkenazi Jews, BRCA1, BRCA2, breast cancer risk, genetic testing
Women with pathogenic BRCA mutations have elevated lifetime risks of breast and ovarian cancer of 40–60% and 20–40%, respectively (1, 2). The prevalence of founder mutations in the BRCA1 (185delAG or 5382insC) or BRCA2 (6174delT) genes is up to 1 in 40 among individuals of Ashkenazi (central and eastern European) Jewish descent (2). Risk management options for mutation carriers include intensive breast cancer screening with mammography and breast MRI (3), risk-reducing surgeries (prophylactic mastectomy, bilateral salpingo-oophorectomy [BSO]) (4), and chemoprevention (5), which have been shown to improve early detection and reduce cancer incidence and mortality. Currently, the U.S. Preventive Services Task Force recommends Ashkenazi Jewish individuals with any first- or second-degree relatives with breast or ovarian cancer be referred for BRCA genetic counseling (6). However, population-based screening in unselected Ashkenazi Jews may identify more mutation carriers.
Despite the potential benefits of BRCA testing, there are still concerns about adverse psychological and social consequences, which may vary by cultural and religious backgrounds. Unique issues may arise among the Orthodox Jewish population due to their adherence to Halacha, Jewish law, or code of ethics. Orthodox Jews represent the largest and most rapidly growing denomination of the Jewish population in New York, but are often underrepresented in genetic studies of Ashkenazi Jews. We conducted a cross-sectional survey to understand knowledge, attitudes, and perceptions of BRCA testing among Orthodox Jewish women.
MATERIALS AND METHODS
We recruited our study population through community-based and religious email listservs in Washington Heights in New York, NY. Inclusion criteria for this study were: (i) women, age ≥18 years, (ii) Orthodox Jews, and (iii) able to give informed consent. The study was approved by the Institutional Review Board at Columbia University Medical Center.
The primary outcome was genetic testing intention/uptake (7). Those who did not answer this question were excluded from the data analyses. We collected data on age, Jewish origin (Ashkenazi, Sephardi, both), Jewish community affiliation (Modern Orthodox, Yeshivish, Chassidish, Lubavitch), highest level of secular and Jewish education, and breast cancer risk factors. To estimate lifetime breast cancer risk, we used the Tyrer-Cuzick model (8), which accounts for age, height, weight, age at menarche and first live birth, menopausal status, hormone replacement therapy use, benign breast disease, family history of breast and ovarian cancer (including age at diagnosis), BRCA genetic test results, and Ashkenazi Jewish ancestry. The questionnaire also included validated measures for health literacy (9), numeracy (10), self-efficacy (11), breast cancer worry (12) and risk perceptions (13), genetic testing knowledge (14), and factors that may influence a decision to undergo BRCA testing (15).
Descriptive statistics were generated for all baseline variables. Frequency distributions between categorical variables were compared using chi-square tests and Fisher’s exact tests when appropriate. To identify independent predictors of genetic testing intention/uptake, multivariable logistic regression models were used. We included variables that were significant (p < 0.15) in the model and then removed variables one at a time if they were nonsignificant (p > 0.10) and did not change any remaining parameter estimates by more than 10%. All analyses were conducted using SAS version 9.3 (SAS Institute, Cary, NC).
RESULTS
Invitations to the online questionnaire were sent to 505 women, 269 (53%) completed the survey, and 243 responded to the genetic testing intention question (Fig. 1). Demographics were not significantly different based upon genetic testing intention/uptake (Table 1). Only one woman was previously diagnosed with breast cancer and no one had ovarian cancer. Among the respondents, 12 (5%) had already been tested for BRCA mutations, 42 (17%) answered “I probably will get tested,” 8 (3%) “I definitely will get tested,” 61 (25%) “I probably will not get tested,” 8 (3%) “I definitely will not get tested,” and 112 (46%) “I haven’t thought about it.”
Figure 1.
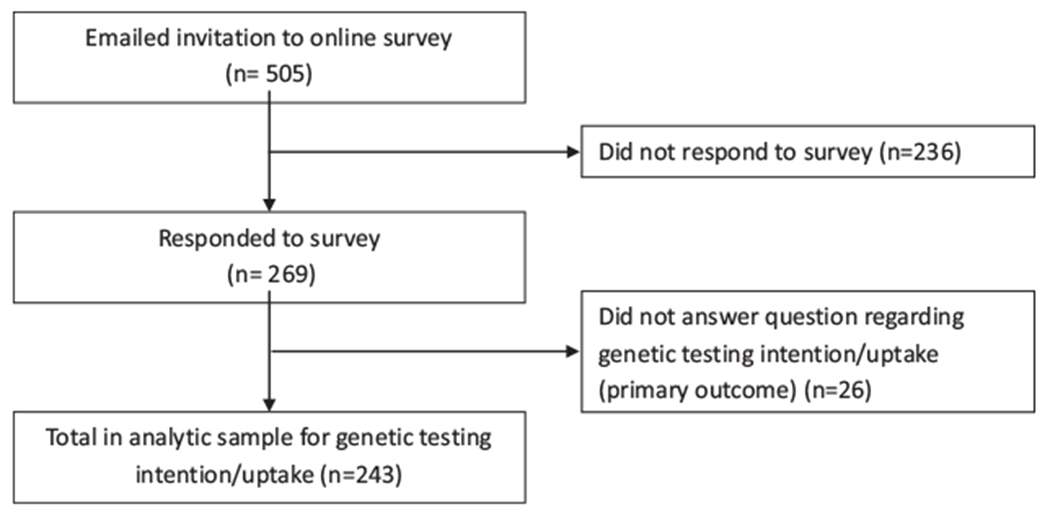
Flow diagram for Orthodox Jewish population.
Table 1.
Baseline Characteristics of Orthodox Jewish Population (N = 243)
| Genetic testing intention/uptake |
|||
|---|---|---|---|
| Yes, N = 62 (25.5%) | No, N = 181 (74.5%) | p-value | |
|
Demographics Age, years | |||
| Median (range) | 24 (19–64) | 25 (19–84) | 0.9558 |
| Jewish origin, N (%) | |||
| Ashkenazi | 47 (87) | 140 (92) | 0.4546 |
| Ashkenazi/Sephardi | 7 (13) | 11 (7) | |
| Jewish community, N (%) | |||
| Modern Orthodox | 36 (71) | 101 (66) | 0.5107 |
| Yeshivish/Chassidish/Lubavitch | 15 (29) | 53 (34) | |
| Highest level of secular education, N (%) | |||
| Master’s/Doctoral degree | 28 (52) | 83 (54) | 0.9067 |
| Some college | 26 (48) | 70 (45) | |
| High school | 0 (0) | 1 (1) | |
| Highest level of Jewish education, N (%) | |||
| Seminary/postseminary | 48 (89) | 144 (94) | 0.3715 |
| None/elementary/high school | 6 (11) | 10 (6) | |
|
Breast cancer risk factors Parous, N (%) | |||
| Yes | 17 (27) | 66 (37) | 0.1859 |
| No | 45 (73) | 114 (63) | |
| Age at first birth, N (%) | |||
| 20–24 years | 8 (47) | 43 (65) | 0.0833 |
| 25–29 years | 7 (41) | 22 (33) | |
| ≥30 years | 2 (12) | 1 (1) | |
| Menopausal status, N (%) | |||
| Premenopausal | 50 (86) | 169 (94) | 0.1318 |
| Postmenopausal | 6 (10) | 9 (5) | |
| Perimenopausal | 2 (3) | 2 (1) | |
| Family history of breast cancer, N (%) | 38 (61) | 67 (37) | 0.0025 |
| Family history of ovarian cancer, N (%) | 5 (8) | 6 (3) | 0.0745 |
| Relative tested positive for BRCA mutation, N (%) | 9 (15) | 4 (2) | 0.0004 |
| Eligible for BRCA genetic testing, N (%) | 40 (65) | 63 (35) | <0.0001 |
|
Validated measures Health literacy | |||
| Median (range, 0 [low]-4 [high]) | 3.3 (0.3–4) | 3.3 (1–4) | 0.5607 |
| High numeracy, N (%) | 46 (87) | 134 (89) | 0.7047 |
| Decision self-efficacy | |||
| (range, 0 [not confident]-4 [very confident]) | 3.5 (1.8–4) | 3 (0–4) | 0.0032 |
| Breast cancer worry | |||
| Median (range, 1 [none]–7 [worry all of the time]) | 2 (1–5) | 1.5 (1–6) | 0.0003 |
| Adequate genetic testing knowledge, N (%) | 37 (61) | 81 (45) | 0.0411 |
| Lifetime breast cancer risk ≥20%, N (%) | 26 (43) | 43 (24) | 0.0052 |
| Perceived lifetime risk of breast cancer, % | |||
| Median (range) | 30 (0–99) | 20 (0–100) | 0.0049 |
| Accuracy of perceived breast cancer risk, N (%) | |||
| Underestimate | 12 (21) | 20 (12) | 0.0016 |
| Accurate (±10%) | 15 (26) | 87 (53) | |
| Overestimate | 31 (53) | 57 (35) | |
In the multivariable logistic regression model (Table 2), respondents were more likely to consider genetic testing with adequate genetic testing knowledge, higher self-efficacy, higher breast cancer risk, and overestimation of risk The three most important factors influencing the decision to undergo BRCA testing (Fig. 2) were “help prevent dying of cancer” (57%), “help prevent getting cancer” (56%), and “effect on my children” (41%).
Table 2.
Multivariable Analysis of Factors Associated with BRCA Genetic Testing Intention/Uptake
| Odds ratio | 95% confidence interval | p-value | |
|---|---|---|---|
| Adequate genetic testing knowledge (Yes versus No) | 2.49 | 1.16–5.33 | 0.019 |
| Decision self-efficacy (range, 0 [not confident]–4 [very confident]) | 1.91 | 1.17–3.11 | 0.010 |
| Breast cancer worry (range, 1 [none]–7 [worry all of the time]) | 1.39 | 0.94–2.04 | 0.096 |
| Actual lifetime breast cancer risk (%) | 1.08 | 1.02–1.14 | 0.008 |
| Accuracy in breast cancer risk perception | |||
| Accurate (referent) | 1.00 | - | - |
| Underestimate | 3.14 | 0.98–10.04 | 0.054 |
| Overestimate | 3.66 | 1.61–8.33 | 0.002 |
Bold indicates statistically significant results (p-values<0.05).
Figure 2.
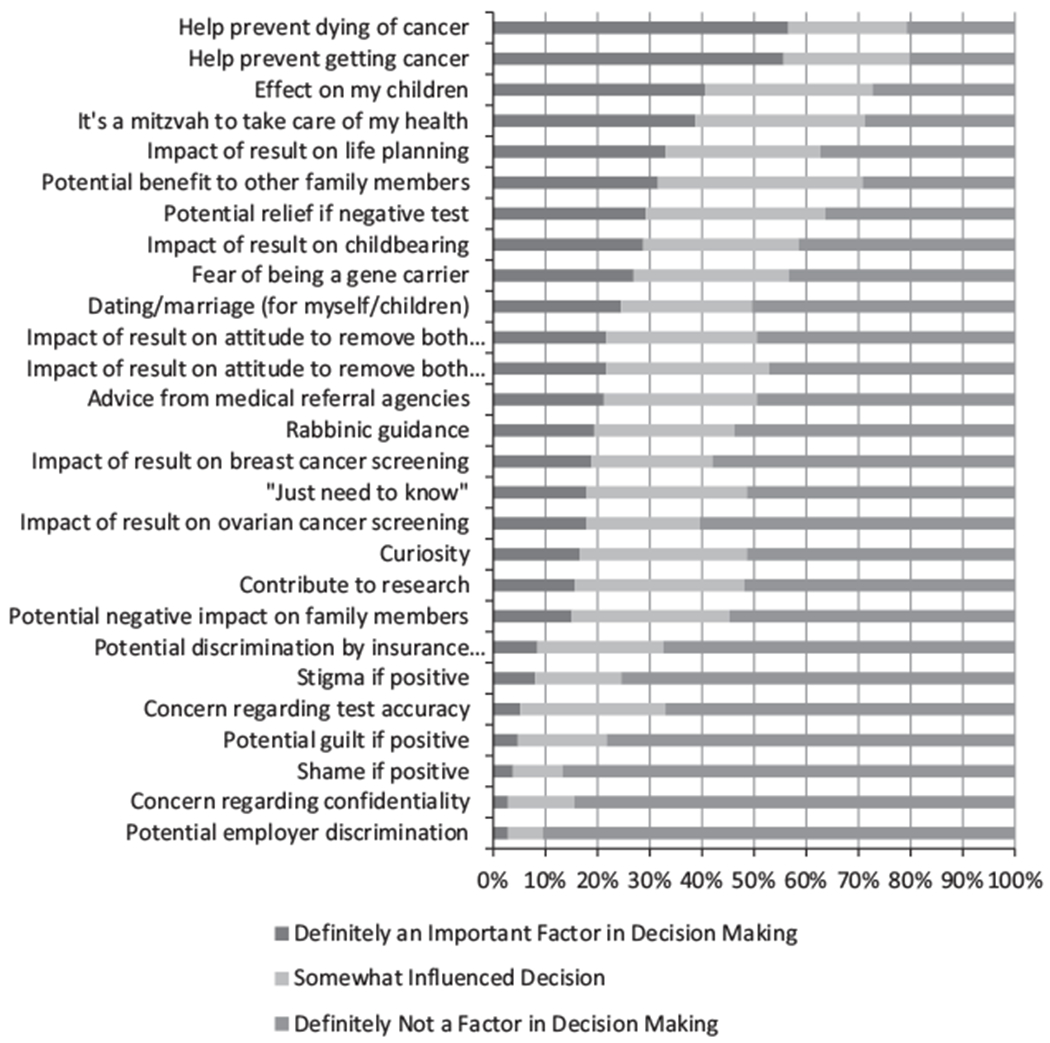
Factors influencing the decision to undergo genetic testing.
DISCUSSION
A key finding from our study is that those with adequate genetic testing knowledge were more likely to consider genetic testing. A prior study found that individuals with greater knowledge about genetic testing were more likely to request BRCA test results (14). In our study, less than half of the women had adequate genetic testing knowledge and over half had inaccurate breast cancer risk perceptions with most overestimating their risk. Although overestimation of breast cancer risk was associated with higher genetic testing intention/uptake, it may also lead to unnecessary cancer worry. Access to educational materials and genetic counseling services may lead to increased genetic testing knowledge and more accurate cancer risk perceptions.
Options for managing cancer risk among BRCA mutation carriers include intensive breast and ovarian cancer screening, risk-reducing surgery, and chemoprevention. In particular, BRCA mutation carriers who underwent risk-reducing BSO had a 79% relative risk reduction in ovarian cancer mortality, 56% reduction in breast cancer mortality, and 77% reduction in allcause mortality (4). Population-based BRCA testing among unselected Ashkenazi Jews can identify more mutation carriers. In a randomized controlled trial of Ashkenazi Jews (16), population-based compared to family history-based screening was able to detect 56% additional BRCA mutation carriers and did not adversely affect short-term psychological/quality of life outcomes. Population-based screening was also shown to be cost-effective (17).
The Orthodox Jewish community is already familiar with population-based genetic screening due to successful testing for autosomal recessive diseases through the Dor Yeshorim program (18). Marriages in the Orthodox Jewish community are often facilitated by shidduchim (matchmaking), in which premarital genetic testing for Tay-Sachs disease is standard practice (18). However, there are unique challenges to testing for BRCA mutations, which are inherited in an autosomal dominant fashion and predispose carriers to adult-onset cancers. Some women may be hesitant to undergo testing due to adverse psychological impact, fear of reducing marriageability, reproductive consequences, and stigma.
Our study is unique in that it had a relatively large population-based sample of Orthodox Jews. In addition, we had a high response rate and used validated measures. A limitation of our study is that the main outcome was genetic testing intention, as only 5% of our survey participants underwent BRCA testing. However, behavioral intention has been found to be highly predictive of actual behavior (19). Second, our study was limited to the Orthodox Jewish community in Washington Heights who were mainly Modern Orthodox, and thus, our findings may not be generalizable to Jewish populations from other areas.
Our study highlights the importance of understanding barriers to BRCA testing in the Orthodox Jewish community, which may be targeted for future interventions. Further research is needed to determine how knowledge about the risks and benefits of BRCA testing are best communicated to women and how this information can be culturally tailored to specific ethnic groups.
Acknowledgments
This publication was supported by the National Center for Advancing Translational Sciences, National Institutes of Health, through grant number UL1 TR000040. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
REFERENCES
- 1.Chen S, Parmigiani G. Meta-analysis of BRCA1 and BRCA2 penetrance. J Clin Oncol 2007;25:1329–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Struewing JP, Hartge P, Wacholder S, et al. The risk of cancer associated with specific mutations of BRCA1 and BRCA2 among Ashkenazi Jews. N Engl J Med 1997;336:1401–8. [DOI] [PubMed] [Google Scholar]
- 3.Warner E, Hill K, Causer P, et al. Prospective study of breast cancer incidence in women with a BRCA1 or BRCA2 mutation under surveillance with and without magnetic resonance imaging. J Clin Oncol 2011;29:1664–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Domchek SM, Friebel TM, Singer CF, et al. Association of risk-reducing surgery in BRCA1 or BRCA2 mutation carriers with cancer risk and mortality. JAMA 2010;304:967–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.King MC, Wieand S, Hale K, et al. Tamoxifen and breast cancer incidence among women with inherited mutations in BRCA1 and BRCA2: National Surgical Adjuvant Breast and Bowel Project (NSABP-P1) Breast Cancer Prevention Trial. JAMA 2001;286:2251–6. [DOI] [PubMed] [Google Scholar]
- 6.Moyer VA. Force USPST. Risk assessment, genetic counseling, and genetic testing for BRCA-related cancer in women: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med 2014;160:271–81. [DOI] [PubMed] [Google Scholar]
- 7.Green MJ, Peterson SK, Baker MW, et al. Effect of a computer-based decision aid on knowledge, perceptions, and intentions about genetic testing for breast cancer susceptibility: a randomized controlled trial. JAMA 2004;292:442–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tyrer J, Duffy SW, Cuzick J. A breast cancer prediction model incorporating familial and personal risk factors. Stat Med 2004;23:1111–30. [DOI] [PubMed] [Google Scholar]
- 9.Chew LD, Bradley KA, Boyko EJ. Brief questions to identify patients with inadequate health literacy. Fam Med 2004;36:588–94. [PubMed] [Google Scholar]
- 10.Lipkus IM, Samsa G, Rimer BK. General performance on a numeracy scale among highly educated samples. Med Decis Making 2001;21:37–44. [DOI] [PubMed] [Google Scholar]
- 11.O’Connor AM. User manual - decision self-efficacy scale [document on the internet] 1995. [modified 2002]. Available at: http://decisionaid.ohri.ca/docs/develop/user_manuals/UM_decision_selfefficacy.pdf (accessed March 17, 2016). [Google Scholar]
- 12.Armstrong K, Micco E, Carney A, Stopfer J, Putt M. Racial differences in the use of BRCA1/2 testing among women with a family history of breast or ovarian cancer. JAMA 2005;293:1729–36. [DOI] [PubMed] [Google Scholar]
- 13.Lipkus IM, Kuchibhatla M, McBride CM, et al. Relationships among breast cancer perceived absolute risk, comparative risk, and worries. Cancer Epidemiol Biomarkers Prev 2000;9:973–5. [PubMed] [Google Scholar]
- 14.Lerman C, Narod S, Schulman K, et al. BRCA1 testing in families with hereditary breast-ovarian cancer. A prospective study of patient decision making and outcomes. JAMA 1996;275:1885–92. [PubMed] [Google Scholar]
- 15.Phillips KA, Warner E, Meschino WS, et al. Perceptions of Ashkenazi Jewish breast cancer patients on genetic testing for mutations in BRCA1 and BRCA2. Clin Genet 2000;57:376–83. [DOI] [PubMed] [Google Scholar]
- 16.Manchanda R, Loggenberg K, Sanderson S, et al. Population testing for cancer predisposing BRCA1/BRCA2 mutations in the Ashkenazi-Jewish community: a randomized controlled trial. J Natl Cancer Inst 2015;107:379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Manchanda R, Legood R, Burnell M, et al. Cost-effectiveness of population screening for BRCA mutations in Ashkenazi Jewish women compared with family history-based testing. J Natl Cancer Inst 2015;107:380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ekstein J, Katzenstein H. The Dor Yeshorim story: community-based carrier screening for Tay-Sachs disease. Adv Genet 2001;44:297–310. [DOI] [PubMed] [Google Scholar]
- 19.Armitage CJ, Conner M. Efficacy of the theory of planned behaviour: a meta-analytic review. Br J Soc Psychol 2001; 40(Pt 4):471–99. [DOI] [PubMed] [Google Scholar]


