FIG 6.
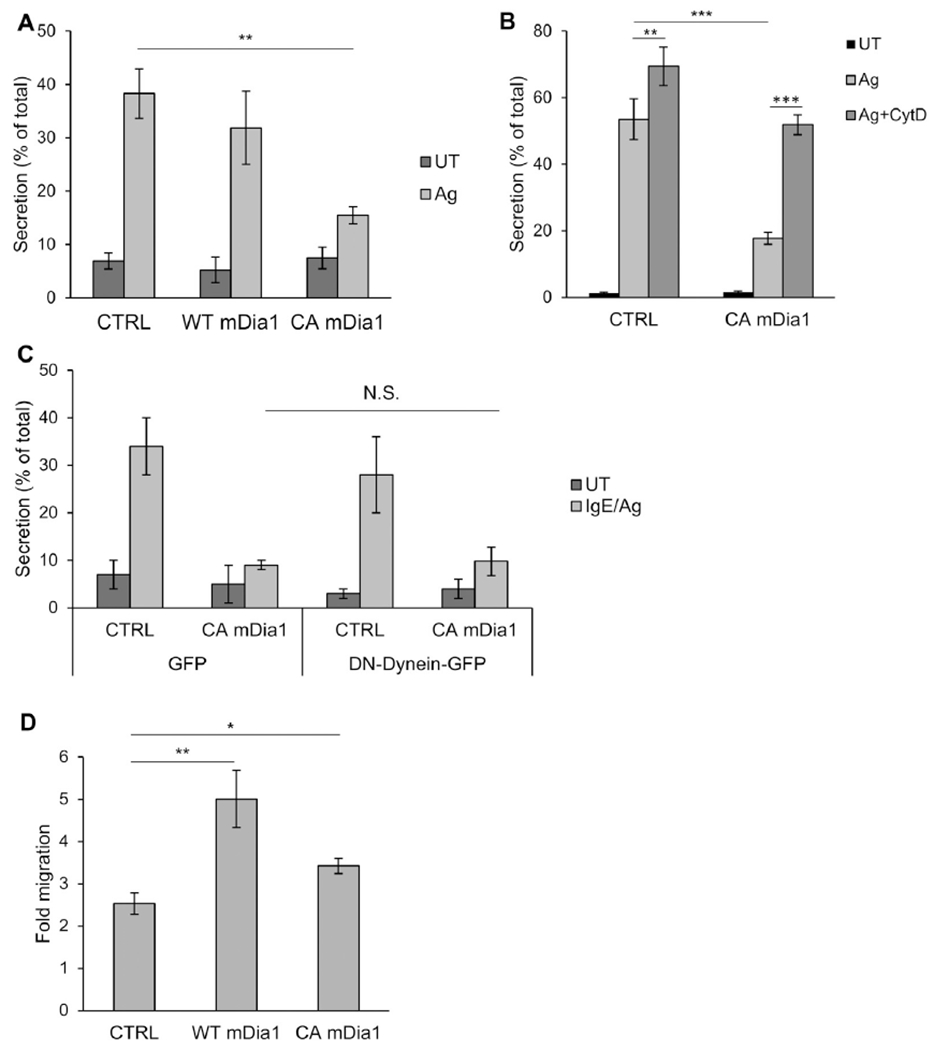
CA mDia1 stimulates migration and inhibits secretion. A, RBL-CXCR1 cells cotransfected with 10 μg of NPY-mRFP and 20 μg of either empty vector (CTRL) or WT or CAmDia1 and sensitized with IgE and were either left untreated (UT) or activated with 50 ng/mLDNP-HSA for 30 minutes (IgE/Ag). NPY-mRFP secretion was measured. Results are means ± SEMs of 4 separate experiments. **P = 2E-3. B, RBL-CXCR1 cells transfected and IgE sensitized as in Fig 6, A, were either left untreated (UT) or pretreated with vehicle or 10 μmol/L CytD for 15 minutes and then activated with 50 ng/mL DNP-HSA for 30 minutes, as indicated. Cells were assayed for NPY-mRFP secretion. Results are means ± SEMs of 3 separate experiments **P = 6.02E-3 and ***P ≤ 6.2E-4. C, RBL-CXCR1 cells were cotransfected with 15 μg of NPY-mRFP and 20 μg of either empty vector and 30 μg of GFP, CA mDia1 and GFP, empty vector and p150glued-CC1-GFP, or CA mDia1 and p150glued-CC1-GFP, as indicated. Cells were IgE sensitized and either left untreated (UT) or activated with 50 ng/ml DNP-HSA for 30 minutes (IgE/Ag). NPY-mRFP secretion was assayed. Results are mean ± SEMs of 3 separate experiments. N.S., Not significant. D, RBL-CXCR1 cells were cotransfected with 10 μg of LifeAct-GFP and 20 μg of either empty vector (CTRL), WT, or CA mDia1, as indicated. Cell migration was assayed in response to 50 ng/mL IL-8. Fold migration was calculated relative to basal migration measured in the absence of IL-8. Results are means ± SEMs of 9 separate experiments. *P = 1.26E-2 and **P = 5E-3.
