Abstract
Pituitary metastases are rare, and metastatic pituitary lesions originating from endometrial adenocarcinoma are extremely rare. These lesions can be mistaken for pituitary adenomas and their diagnosis can be very difficult. Pituitary metastases mostly affect the posterior lobe and patients may develop diabetes insipidus. Patients with endometrial cancer complicated with diabetes, including poor glycemic control, may also suffer from thirst, making it more difficult to diagnose diabetes insipidus. A 68-year-old woman who was being followed-up for primary endometrial adenocarcinoma was admitted for gradually worsened polyuria and polydipsia. Her laboratory findings were compatible with diabetes insipidus. Magnetic resonance imaging revealed thickening of the pituitary stalk, involvement of the superior pituitary gland, and disappearance of hyperintensity in the posterior lobe, indicating pituitary metastasis. Increased urine output and oral fluid intake in a patient with a diagnosis of carcinoma may indicate possible pituitary metastasis, and the hormonal insufficiency should be corrected to improve the patient’s quality of life.
Keywords: Diabetes insipidus, pituitary, endometrial adenocarcinoma, metastasis, urine output, thirst
Introduction
Metastasis in the pituitary gland is a rare complication of systemic cancers. Benjamin first reported a case of pituitary metastasis in 1857, detected during autopsy of a patient with disseminated melanoma, since when there have been numerous autopsy reports and clinical series regarding pituitary metastasis. Cancers of various origins may metastasize to the pituitary gland, most notably, breast and lung cancers.1 However, there have been few reports of the metastasis of malignant uterine tumors to the pituitary gland.
Pituitary metastasis usually affects the posterior lobe of the hypophysis, and most pituitary metastases are asymptomatic and are detected incidentally on imaging.2 However, diabetes insipidus is the most common symptom of pituitary metastases, and may be the initial clinical finding of an undiagnosed malignancy.3
We present the case of a patient with diabetes and endometrial adenocarcinoma with metastasis to the pituitary gland. We report on her clinical presentation and imaging diagnosis, and discuss the current understanding of the pathogenesis and treatment strategies associated with pituitary metastases. We also carried out a search of the PubMed database using the keywords “pituitary”, “endometrial adenocarcinoma”, and “metastasis” up to July 2018, and reviewed the relevant publications.
Case report
A 68-year-old woman was admitted with complaints of increasing polyuria, polydypsia, weight loss, and fatigue. She had been diagnosed with type II diabetes 5 years and 6 months ago, in 2011, and with endometrial adenocarcinoma in November 2016. After the diagnosis, she underwent immediate total hysterectomy, bilateral adnexectomy, and lymphadenectomy. However, her symptoms of polyuria, polydipsia, weight loss, and fatigue reappeared after the operation and worsened over time. Her symptoms were thought to be due to poor glycemic control, but were not relieved even when her blood glucose level was stable. She had approximately 7 L of urinary output and oral fluid intake per day.
Laboratory investigations revealed a serum sodium level of 165.5 mmol/L (normal range 135–145 mmol/L), glucose 6.4 mmol/L, and normal levels of blood potassium, creatinine, and urea nitrogen (Table 1). Her erythrocyte sedimentation rate was 95 mm/hour. Hormone profiling revealed serum prolactin 96.87 ng/mL (normal range 2.64–13.13 ng/mL), growth hormone 0.684 ng/mL (normal range 0.01–3.61 ng/mL), normal postmenopausal follicle-stimulating hormone and luteinizing hormone, estradiol 15 pg/mL (normal postmenopausal range 20–40 pg/mL), and normal adrenocorticotrophic hormone and thyroid function. Notably, her urine specific gravity was <1.005 g/mL. Urinary and plasma osmolalities were 101 and 324 mOsm/kg H2O, respectively, compared with a urinary specific gravity 2 months earlier of 1.010 g/mL. A water deprivation test indicated central diabetes insipidus. Prescription of desmopressin (0.1 mg/day) reduced her urinary output to 3 L/day and her oral fluid intake to 4 L/day. She had been amenorrheic for 14 years and endocrinological test results were compatible with the menopause.
Table 1.
Laboratory findings.
| Parameter | Content | Normal range |
|---|---|---|
| Sodium | 165.5 | 135–145 mmol/L |
| Potassium | 3.5 | 3.5–4.5 mmol/L |
| Creatinine | 45.3 | 62–115 µmol/L |
| Luteinizing hormone | 12.1 | 10.87–58.64 mIU/mL |
| Estradiol | 15,1 | 20–40 pg/mL |
| Adrenocorticotrophic hormone | 9.9 | 9–46 pg/mL |
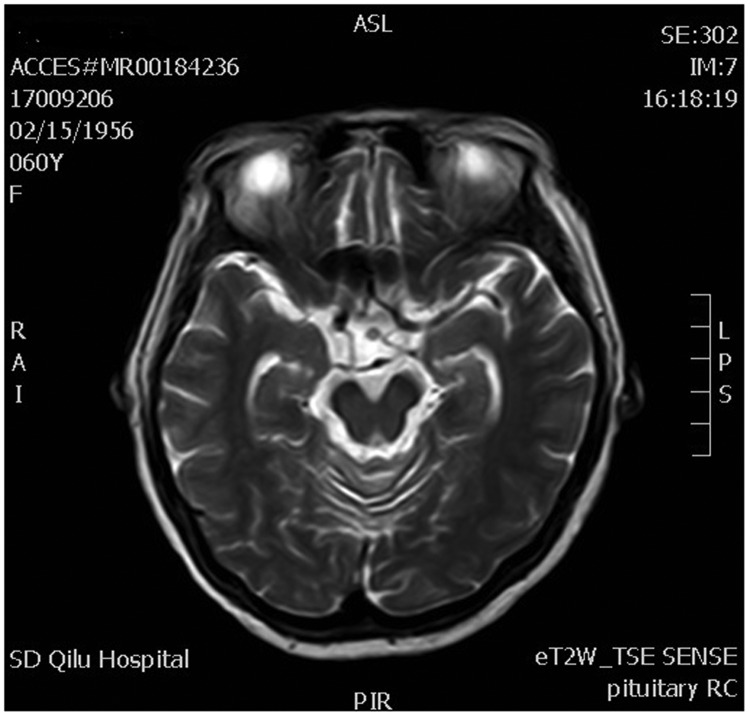
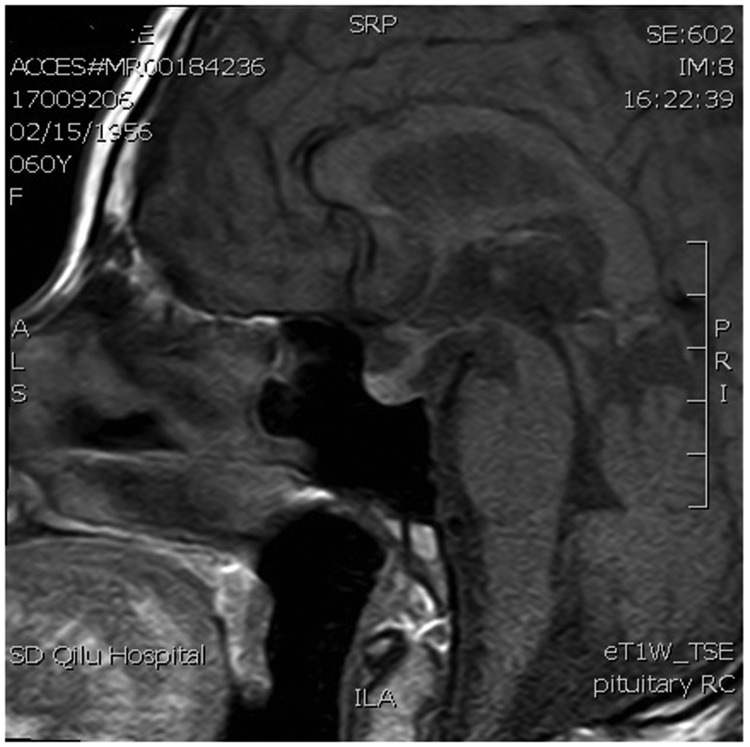
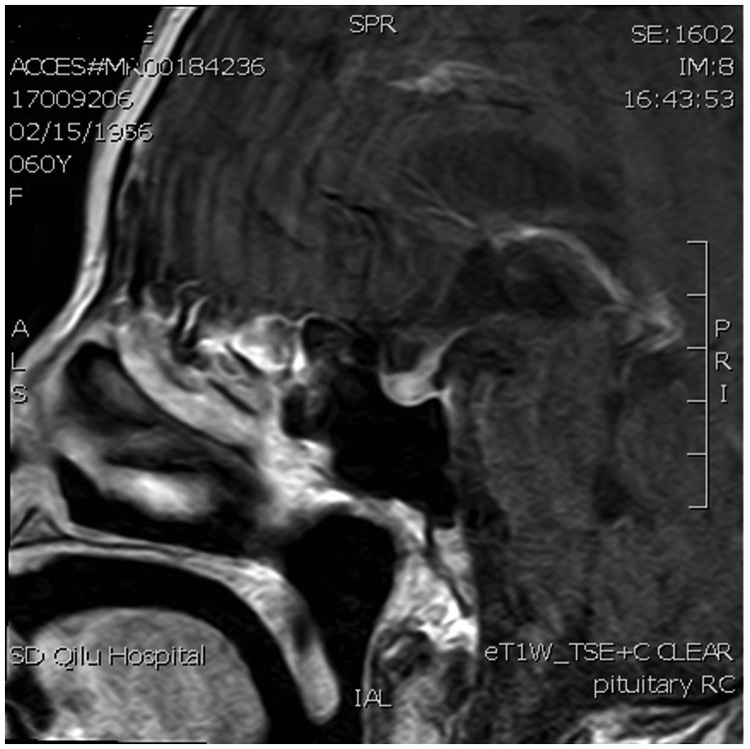
Postoperative computed tomography scan revealed no abnormalities in the chest and abdomen, and breast examination with molybdenum target showed no signs of cancer. Magnetic resonance imaging (MRI) of the pelvic cavity revealed no metastases and lumbar MRI showed no vertebral bone metastases. Postoperative MRI of the brain revealed high signal in the pituitary stalk but no signs of a metastatic site. T2-weighted MRI demonstrated thickening of the pituitary stalk (Figure 1), and T1-weighted MRI revealed thickening of the pituitary stalk, involvement of the superior border of the pituitary gland, and disappearance of hyperintensity in the posterior lobe (Figure 2). Enhanced T1-weighted MRI showed that the thickened pituitary stalk was significantly and continuously enhanced, and the superior border of the pituitary gland was involved, with heterogeneously enhanced signal intensity in the pituitary gland after administration of gadolinium (Figure 3). There was no obvious sign of an anterior pituitary tumor.
Figure 1.
Magnetic resonance T2-weighted image (T2W1) showing thickening of the pituitary stalk (arrow).
Figure 2.
Magnetic resonance T1-weighted image (T1W1) showing thickening of the pituitary stalk, involvement of the superior border of the pituitary gland, and disappearance of hyperintensity in posterior lobe (arrow).
Figure 3.
Gadolinium-enhanced magnetic resonance T1-weighted image showing significantly and continuously enhanced thickened pituitary stalk, involvement of the superior border of the pituitary gland, and heterogeneously enhanced signal intensity in the pituitary gland (arrow).
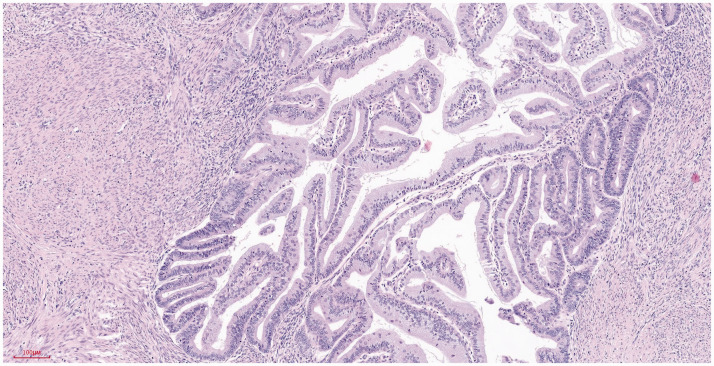
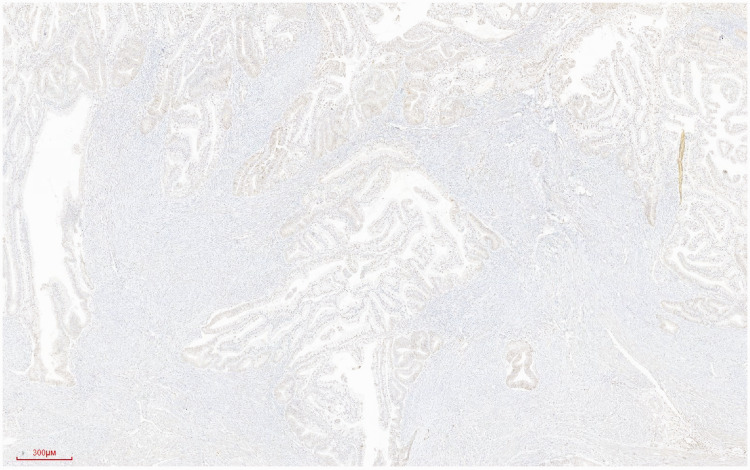
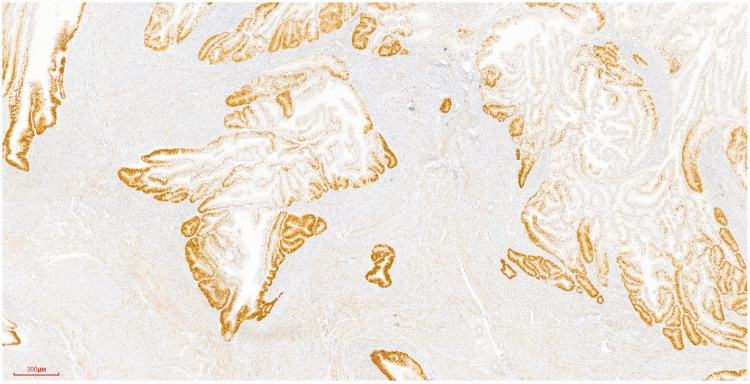
Postoperative primary tumor pathology showed a 6 × 5 × 1.4 cm tumor in the uterus, elevated over the endometrium, occupying >50% of the total volume of the uterine cavity, and invading the superficial muscularis to about half the depth of the muscle wall (Figure 4). Histologically, the tumor was a grade 1 diffuse endometrioid adenocarcinoma, without typical intravascular tumor thrombus and nerve invasion (Figures 5 and 6).
Figure 4.
Highly differentiated endometrioid adenocarcinoma infiltrating the superficial muscularis. Hematoxylin and eosin staining. Magnification ×100.
Figure 5.
Immunohistochemical staining of endometrioid adenocarcinoma showing the expression of p53 in the nucleus. Streptavidin–peroxidase conjugate staining using monoclonal antibody against p53 (Maixin Biotech, Fuzhou, China). Magnification ×40.
Figure 6.
Immunohistochemical staining of endometrioid adenocarcinoma showing progesterone receptor expression in the nucleus. Streptavidin–peroxidase conjugate staining using monoclonal antibody against progesterone receptor (Maixin Biotech). Magnification ×40.
We planned radiotherapy and chemotherapy, but the patient refused these treatments and died 86 days after operation.
This report was prepared according to the CARE guidelines and the study was approved by the Research Ethics Review Committee of Liaocheng People’s Hospital (Approval no. LPHL-212). Patient consent was not obtained and her identity has been removed from the report; however written consent was obtained from the patient’s carer.
Discussion
Although malignant tumors metastasizing into the pituitary fossa are not infrequent events in autopsy series and may occur in 3% to 5% of patients with pituitary adenoma, pituitary metastases are rarely diagnosed antemortem.4 To help us understand the current case, we searched the PubMed database using the keywords “pituitary”, “endometrial adenocarcinoma”, and “metastasis” up to July 2018 and reviewed the relevant publications. Pituitary metastasis of endometrial adenocarcinoma is very rare, with only three reported cases described to date.5–7 In the present case, a history of preexisting endometrial adenocarcinoma, typical clinical onset, and radiologic findings supported the diagnosis of pituitary metastasis of endometrial adenocarcinoma.
There was no reported difference in the incidence of pituitary metastasis between males and females, but it was most commonly detected in elderly patients during their sixth or seventh decades.8 Accordingly, the current patient was 68 years old. The most likely sources in women are breast, lung, and stomach cancers, while those in men are lung, prostate, and bladder cancers. Less common sources are lymphoma, leukemia, plasmacytoma, melanoma, and renal cell carcinoma. Among 201 patients with pituitary metastases, Habu et al.9 reported that lung cancer was the most frequent primary tumor among 109 men, followed by kidney, lymphatic, liver, colorectal, and prostate cancers, while breast cancer was the most frequent source among the 92 women, followed by lung, colorectal, kidney, stomach, and uterine tube cancer.
The location of the pituitary metastases involved the posterior pituitary, either alone or in combination with the anterior pituitary in 84.6% of cases (170/201), and the anterior pituitary in 15.4% (31/201).10 In addition, metastasis to the anterior pituitary typically originates via spread from primary tumors in the posterior pituitary. Tumors may metastasize to the pituitary either by hematogenous spread or by direct invasion from the skull base. Hematogenous spread can occur either from the hypophyseal arteries, in which case the posterior pituitary is usually involved first, or via the portal system. The pituitary stalk is supplied by the superior pituitary artery and the posterior lobe by the inferior pituitary artery. In the current case, the pituitary stalk was thickened, and there was no sign of an anterior pituitary tumor.
About 7% to 50% of pituitary metastases are symptomatic,4,10,11 and sellar metastasis may be the initial manifestation of an unknown primary tumor.4,11 Max et al.6 reviewed the English language literature from 1914 to 1979 and identified 28 symptomatic pituitary metastases in 178 metastatic deposits, with clinical features including diabetes insipidus in 71%, ophthalmoplegia in 15%, anterior pituitary insufficiency in 7%, and visual loss in 7%. Up to 15% of adult cases of central diabetes insipidus are the result of metastatic involvement,12 and the development of diabetes insipidus, which is a rare manifestation of a pituitary adenoma regardless of size, should raise the possibility of metastatic disease.11 Unlike typical cases of pituitary metastasis, the current patient presented with typical symptoms of diabetes insipidus but no signs of pituitary compression, because the lesion was small.
Metastasis to the posterior pituitary is recognized to cause diabetes insipidus, while metastasis to the anterior pituitary is believed to cause anterior pituitary insufficiency. Pituitary metastasis can cause a variety of hormonal abnormalities, including hyperprolactinemia in 6.3% of cases, as a result of pituitary stalk compression.1 Prolactinoma should be suspected in patients with prolactin levels >200 ng/mL, while pituitary metastasis is typically associated with a prolactin level <149.2 ng/mL.1 In the current patient, her prolactin level was elevated but did not exceed the limit that could allow the differentiation between pituitary metastasis and pituitary adenoma.
Regarding hormone profiles, the patient’s growth hormone and adrenocorticotrophic hormone levels were within normal limits and she had no hypothyroidism or hypogonadism, but did have hyperprolactinemia. On the basis of the hormone analysis and the presence of diabetes insipidus, we suspected that the pituitary metastasis in the current patient involved both the pituitary stalk and the posterior pituitary. This speculation was confirmed by MRI, although no obvious occupying tissue was found in the posterior pituitary gland.
Neuroradiological findings of metastasis in the pituitary gland are less specific for a diagnosis than the clinical presentations. Thickening of the pituitary stalk, sclerosis of the surrounding sella turcica, and invasion of the cavernous sinus may indicate a secondary lesion.13 Constriction at the diaphragma sellae was seen in all four previously reported cases of pituitary metastasis,14 and increased signal intensity in the contiguous hypothalamus on T2-weighted MRI was frequently observed in these metastases.15 Edema along the optic chiasm and tract was first described in a case of craniopharyngioma and was subsequently seen in other sellar lesions, including pituitary metastasis, but was rarely seen in pituitary adenoma.16 Bony changes, such as the erosion of surrounding bony structures and sellar enlargement, are also well-known findings.1
Pituitary adenomas are isointense on T1-weighted and T2-weighted MRI, and show homogenous or heterogeneous gadolinium enhancement. The only distinguishing feature between adenoma and pituitary metastasis on MRI is hyperintensity on T2-weighed MRI.17 The most important differentiating characters are derived from the fact that adenomas are slow-growing tumors that expand the sella, destroy the diaphragm, and lead to bony erosion. In the current case, MRI revealed thickening of the pituitary stalk, involvement of the superior border of the pituitary gland, disappearance of hyperintensity in the posterior lobe, and heterogeneous enhancement after administration of gadolinium, but no bone erosion or diaphragm damage.
The management of pituitary metastasis in patients with known primary lesions often involves palliative care. The possible treatment modalities include complete tumor resection, surgical decompression, sellar radiation therapy including radiosurgery and fractionated radiotherapy, and systemic chemotherapy and/or intrathecal chemotherapy. We planned radiotherapy and chemotherapy in the current patient, but the patient refused these treatments. Although life expectancy is limited in patients with advanced cancer, hormonal insufficiency should be corrected to improve their quality of life.
The prognosis of patients with pituitary metastasis is generally poor, with a reported mean survival time of 6 to 7 months. However, a few studies have reported exceptionally long survival. The current patient died 86 days after operation.
Notably, a definitive diagnosis of pituitary metastasis requires histologic examination of specimens obtained by transsphenoidal surgery or biopsy, while the current patient was diagnosed based on their neuroimaging findings, clinical presentation, and clinical course, rather than on histological evidence. We therefore cannot rule out the possibility that the tumor was a pituitary incidentaloma. However, the endometrial adenocarcinoma was biologically aggressive and at an advanced stage, and the patient only survived for a short time after detection of the pituitary tumor, and these poor conditions thus precluded a biopsy in this case. Recent reports of pituitary metastases have also included a high proportion of patients with non-definite diagnoses because of the lack of an autopsy.9,18,19
Conclusions
Diabetes insipidus and hormone profiles are key to recognizing the existence of pituitary metastasis, and patients with primary endometrial adenocarcinoma presenting with diabetes insipidus should be evaluated for possible pituitary metastasis. Increased urine output and oral fluid intake in a patient with a diagnosis of carcinoma should alert the physicians to the possibility of pituitary metastasis.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Ethics and consent statements
This study was approved by the Research Ethics Review Committee of Liaocheng People’s Hospital (Approval no. LPHL-212) and written consent was obtained from the patient’s carer.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
ORCID iD
Hongquan Du https://orcid.org/0000-0002-9183-3838
References
- 1.Komninos J, Vlassopoulou V, Protopapa D, et al. Tumors metastatic to the pituitary gland: case report and literature review. J Clin Endocrinol Metab 2004; 89: 574–580. DOI: 10.1210/jc.2003-030395. [DOI] [PubMed] [Google Scholar]
- 2.Rajput R, Bhansali A, Dutta P, et al. Pituitary metastasis masquerading as non-functioning pituitary adenoma in a woman with adenocarcinoma lung. Pituitary 2006; 9: 155–157. DOI: 10.1007/s11102-006-8326-0. [DOI] [PubMed] [Google Scholar]
- 3.Granata A, Figura M, Gulisano S, et al. [Central diabetes insipidus as a first manifestation of lung adenocarcinoma]. Clin Ter 2007; 158: 519–522. [Article in Italian]. [PubMed] [Google Scholar]
- 4.Post K, McCormick P, Hays A, et al. Metastatic carcinoma to pituitary adenoma: report of two cases. Surg Neurol 1988; 30: 286–292. [DOI] [PubMed] [Google Scholar]
- 5.Lieschke GJ, Tress B, Chambers D. Endometrial adenocarcinoma presenting as pituitary apoplexy. Aust N Z J Med 1990; 20: 81–84. DOI: 10.1111/j.1445-5994.1990.tb00379.x. [DOI] [PubMed] [Google Scholar]
- 6.Max MB, Deck MD, Rottenberg DA. Pituitary metastasis: incidence in cancer patients and clinical differentiation from pituitary adenoma. Neurology 1981; 31: 998–1002. DOI: 10.1212/wnl.31.8.998. [DOI] [PubMed] [Google Scholar]
- 7.Thomas JE, Yoss RE. The parasellar syndrome: problems in determining etiology. Mayo Clin Proc 1970; 45: 617–623. [PubMed] [Google Scholar]
- 8.Hsiao C, Wang C, Chung M, et al. Diabetes insipidus due to pituitary metastasis in a woman with lung adenocarcinoma: a case report. Cent Eur J Med 2011; 6: 475–479. [Google Scholar]
- 9.Habu M, Tokimura H, Hirano H, et al. Pituitary metastases: current practice in Japan. J Neurosurg 2015; 123: 998–1007. DOI: 10.3171/2014.12.JNS14870. [DOI] [PubMed] [Google Scholar]
- 10.McCormick PC, Post KD, Kandji AD, et al. Metastatic carcinoma to the pituitary gland. Br J Neurosurg 1989; 3: 71–79. DOI: 10.3109/02688698909001028. [DOI] [PubMed] [Google Scholar]
- 11.Branch CL JrandLaws ER Jr.. Metastatic tumors of the sella turcica masquerading as primary pituitary tumors. J Clin Endocrinol Metab 1987; 65: 469–474. DOI: 10.1210/jcem-65-3-469. [DOI] [PubMed] [Google Scholar]
- 12.Esiri M. Russell and Rubinstein’s pathology of tumors of the nervous system. Sixth edition. J Neurol Neurosurg Psychiatry 2000; 68: 538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fassett DR, Couldwell WT. Metastases to the pituitary gland. Neurosurg Focus 2004; 16: E8. [PubMed] [Google Scholar]
- 14.Schubiger O, Haller D. Metastases to the pituitary–hypothalamic axis. An MR study of 7 symptomatic patients. Neuroradiology 1992; 34: 131–134. DOI: 10.1007/bf00588159. [DOI] [PubMed] [Google Scholar]
- 15.McCutcheon IE, Kitagawa RH, Sherman SI, et al. Adenocarcinoma of the salivary gland metastatic to the pituitary gland: case report. Neurosurgery 2001; 48: 1161–1165; discussion 1165-1166. DOI: 10.1097/00006123-200105000-00044. [DOI] [PubMed] [Google Scholar]
- 16.Saeki N, Murai H, Kubota M, et al. Oedema along the optic tracts due to pituitary metastasis. Br J Neurosurg 2001; 15: 523–526. DOI: 10.1080/026886901317195482. [DOI] [PubMed] [Google Scholar]
- 17.Kramer CK, Ferreira N, Silveiro SP, et al. Pituitary gland metastasis from renal cell carcinoma presented as a non-functioning macroadenoma. Arq Bras Endocrinol Metabol 2010; 54: 498–501. DOI: 10.1590/s0004-27302010000500011. [DOI] [PubMed] [Google Scholar]
- 18.Iwai Y, Yamanaka K, Honda Y, et al. Radiosurgery for pituitary metastases. Neurol Med Chir (Tokyo) 2004; 44: 112–116; discussion 117. DOI: 10.2176/nmc.44.112. [DOI] [PubMed] [Google Scholar]
- 19.Kano H, Niranjan A, Kondziolka D, et al. Stereotactic radiosurgery for pituitary metastases. Surg Neurol 2009; 72: 248–255; discussion 255-246. DOI: 10.1016/j.surneu.2008.06.003. [DOI] [PubMed] [Google Scholar]