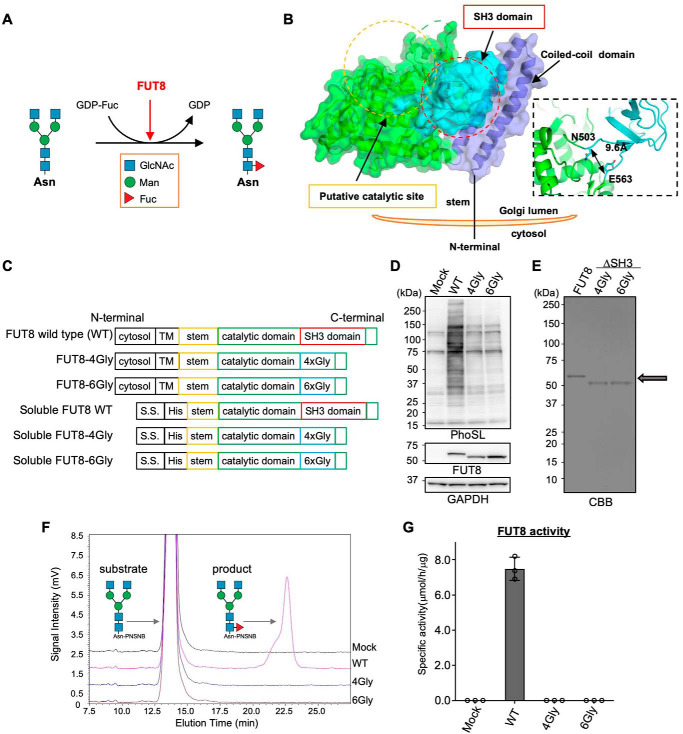
Figure 1.
Core fucosylation activity of FUT8 WT and FUT8ΔSH3. A, schematic model of core fucosylation by FUT8. B, the three-dimensional structure of human FUT8 luminal domain (left) and domain structures of FUT8 WT, FUT8ΔSH3, and soluble forms of FUT8. C, plasmid constructs used in this study. D, FUT8 WT or FUT8ΔSH3 was expressed in HEK293 FUT8KO cells. The proteins in cell lysates were separated by SDS-PAGE and then analyzed by Western blotting with anti-FUT8 (sheep Ab) and GAPDH Abs or lectin blotting with PhoSL. E, soluble FUT8 WT and ΔSH3 were expressed in COS7 cells and purified from the media using a Ni2+ column. An arrow indicates the positions of purified FUT8 WT and ΔSH3, which were visualized by CBB staining. F, purified FUT8 WT and ΔSH3 were reacted with the oligosaccharide acceptor (GnGn-bi-Asn-PNSNB) and GDP-Fuc, and the acceptor substrate and the product were separated using HPLC. G, the specific activity of FUT8, calculated by the peak area in F (n = 3, mean ± S.D. (error bars)). The measurements were taken from triplicates of one sample.