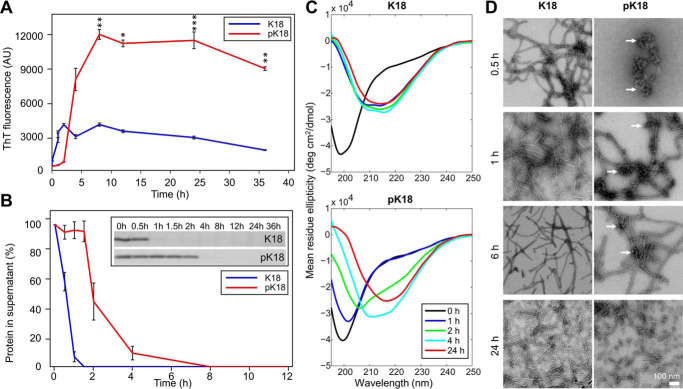
Figure 6.
Comparison of the aggregation behavior of nonphosphorylated and phosphorylated K18. 10 μm non- and phosphorylated Tyr → Phe Tau mutants were incubated for 48 h at 37 °C under shaking conditions, in the presence of 2.5 μm heparin and the extent of aggregation was monitored by ThT fluorescence (A), sedimentation assays (B), CD (C), and EM (scale bar = 100 nm for all images) (D). Together, the results from these different assays show that phosphorylation at Tyr-310 has an inhibitory role during the nucleation phase of K18 aggregation, and delays formation of β-sheet–rich K18 fibrils. In A, two-way ANOVA with Tukey's multiple comparisons test, significance values are indicated by: *, p < 0.05; **, p < 0.01; ***, p ≪ 0.001. B, at several times points, an aliquot of the aggregation reaction was taken and centrifuged. The supernatants were run on SDS-PAGE (inset). The supernatant band intensity from four independent experiments was quantified and reported as the percent of protein remaining in the supernatant (mean ± S.D.).