Figure 1.
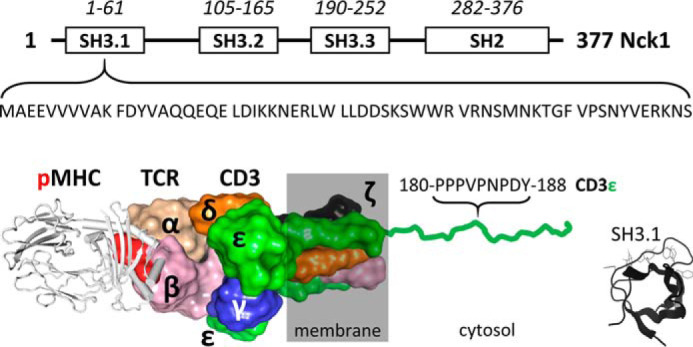
Human Nck1 domain organization and interaction of its N-terminal SH3.1 domain with the CD3ϵ subunit of the T cell receptor. Top, sequence boundaries of the three SH3 domains and the C-terminal SH2 domain of Nck. The sequence of the SH3.1 domain interacting with CD3ϵ is noted. Bottom, sketch of the peptide-MHC/TCR interaction (based on a superposition of PDB entries 2CKB and 6JXR). The TCR and CD3 subunits are labeled. Cytosolic tails are omitted for clarity except for one of the CD3ϵ subunits to highlight the PRS that binds to Nck1-SH3.1. Tyr-188 in the PRS (red, boldface type) is the first tyrosine in the ITAM of CD3ϵ. The Nck1/CD3ϵ interaction can be mimicked in vitro by the isolated Nck1-SH3.1 domain (residue range 4–59 is sufficient; inset) and CD3ϵ-derived peptides.
