Figure 6.
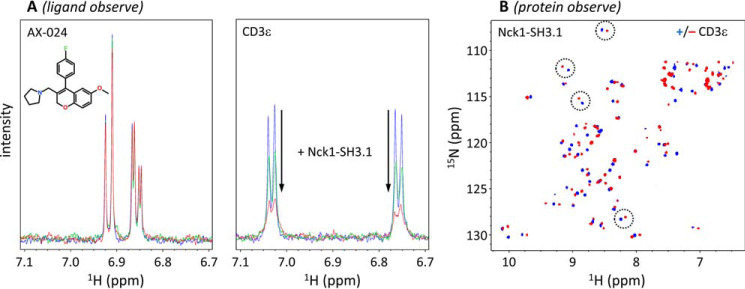
NMR detects CD3ϵ but not AX-024 binding to Nck1-SH3.1. A, ligand-observed NMR. Left, aromatic AX-024 resonance signals (50 μm solution) do not show any spectral changes upon the addition of monomeric (red) and dimeric (green) Nck1-SH3.1 (10 μm; residues 1–61). Right, in contrast, substoichiometric amounts of Nck1-SH3.1 lead to line broadening of CD3ϵ peptide RGQNKERPPPVPNPDY Tyr-188 Hδ and Hϵ resonances, indicating fast-exchange binding. The addition of monomeric (red) Nck1-SH3.1 shows strong line broadening (i.e. binding to the peptide), in contrast to dimeric Nck1-SH3.1 (green), which shows much less line broadening. B, protein-observed 1H-15N HSQC NMR confirms Nck1-SH3.1 (residues 4–59) binding to the CD3ϵ peptide by distinct chemical shift perturbations (representative pairs ± CD3ϵ are circled), as also reported in Ref. 10. For this experiment, the monomeric form of SH3.1 was used. The blue and red spectra are without and with CD3ϵ peptide, respectively.