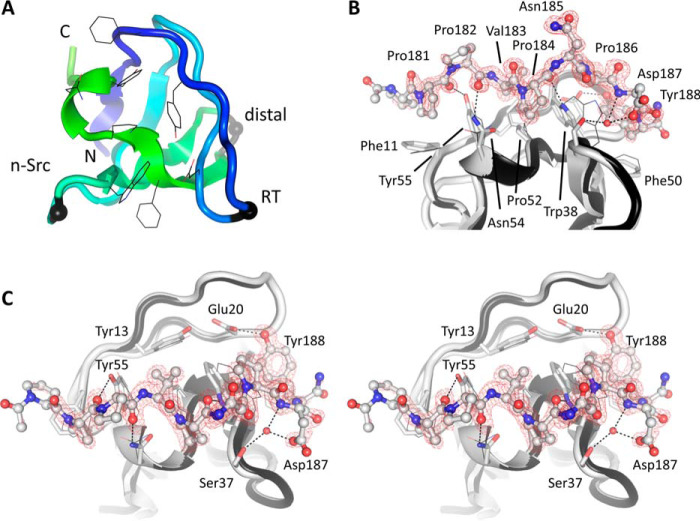
Figure 7.
Crystal structure of the Nck1-SH3.1 domain and its complex with CD3ϵ peptide. A, the apo-structure of Nck1-SH3.1 is shown as a ribbon with the peptide-binding side chains drawn as lines. Three distinctive loop regions of SH3 domains are indicated by black spheres. B, Nck1-SH3.1 in complex with the CD3ϵ peptide PPPVPNPDY. The omit electron density, contoured as a red mesh at 3 RMSD, shows that the first and last residues of the peptide are less well-defined than the central part, implying flexibility. C, the cross-eyed stereo view is rotated by 90° about the horizontal axis compared with B. The largest structural changes in the Nck1-SH3.1 domain upon peptide binding are side-chain adjustments and inclusion of a water molecule (red sphere) that is absent in the apo-Nck1-SH3.1 structure. Atoms between SH3.1 and the peptide in hydrogen-bond geometry are connected by dashed lines. Note that a phosphorylated Tyr-188 would not fit into the binding site of the SH3.1 domain.