Figure 8.
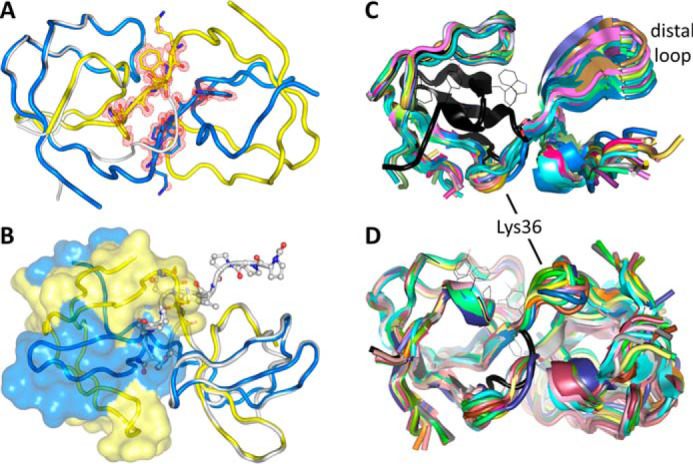
Domain-swapped Nck1-SH3.1. A, the domain swap at Lys-36 in the n-Src loop. Omit electron density is shown in red at the 3 RMSD level for the sequence 36KSWW39. The main chains follow an anti-parallel β-sheet in the dimer (shown as yellow and blue ribbons). The monomeric SH3.1 is drawn as a gray ribbon for reference. B, one half of the swapped dimer is shown as a transparent surface. The CD3ϵ peptide-Nck1-SH3.1 complex is superimposed onto the other half of the dimer. The peptide, drawn as a ball-and-stick model, clashes with the surface, indicating no or only residual affinity for the complex. C and D, structural variability of the dimers. The nonswapped, monomeric Nck1-SH3.1 domain is shown in black as a reference. C, 38 individual Nck1-SH3.1 chains from crystal structures were superimposed on the N-terminal half of the SH3.1 domains, showing the differences for the second half. D, superposition of 21 individual dimers onto one of the protomers shows how the structural variability of the domain-swapped chains results in very different conformations of the dimers.
