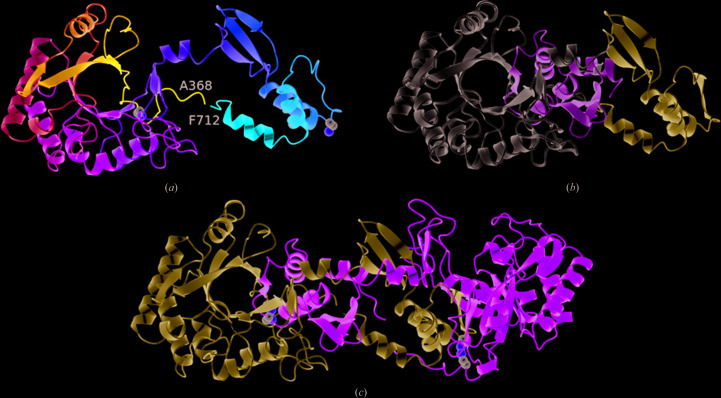
Figure 3.
Domain swapping in the chitinase domain of CotE. (a) Ribbon rendering of the chitinase domain in the P6122 crystal structure. The chain is colour-ramped as in Fig. 1 ▸(a), and the Cα atoms and side chains of cysteines 376 and 670 are shown as spheres. The C-terminal residues 627–712 extend away from the β-barrel so as to pack onto and complete the β-barrel of a crystallographic symmmetry mate, as shown in (c), where the two subunits of the domain-swapped dimer are coloured light blue and green, respectively. The eighth strand of each β-barrel is provided by the partner subunit. (b) A juxtaposition of the C-terminal swapped domains (residues 627–712) in the P21 and P6122 crystal forms, coloured light green and ice blue, respectively, relative to the β-barrel domain (in white) following the superposition of residues 358–623 is shown.