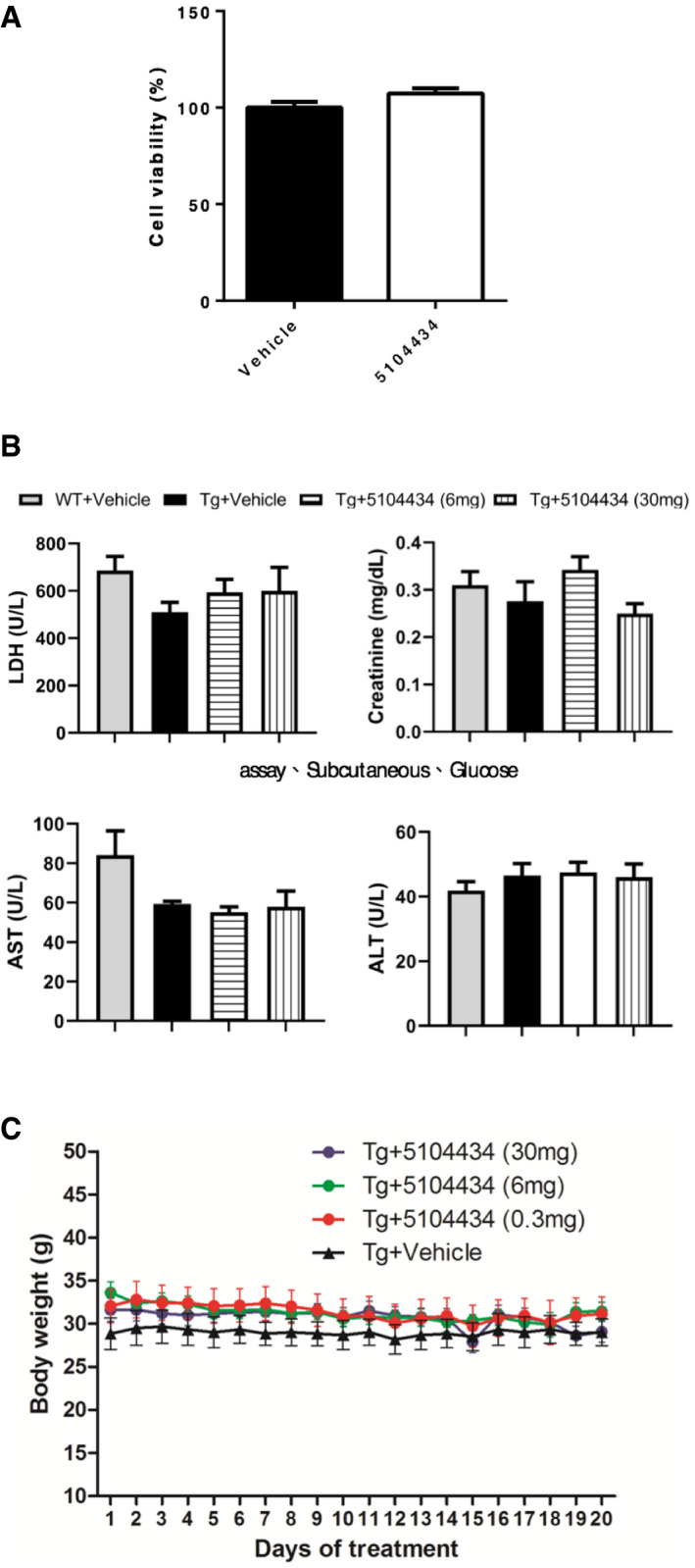
Figure EV5. Evaluation of the pharmaceutical side effects with respect to cell viability in cell model, hepatotoxicity, renal toxicity, and body weight after 1 month of compound 5104434 treatment in animal model.
-
ACell viability was detected under 5104434 treatment for 72 h in 293T cell line. N = 3 independent experiments.
-
BEvaluation of the serum chemistry profile including lactate dehydrogenase (LDH), creatinine, aspartate transaminase (AST), and alanine transaminase (ALT) in both WT and FTLD‐TDP Tg mice treated with vehicle, 6 mg/kg/day, and 30 mg/kg/day of 5104434. N = 10, 5, 5, 15 mice in each group.
-
CRecords of body weight in vehicle and 5104434‐treated FTLD‐TDP Tg mice. N = 5 mice per group.
