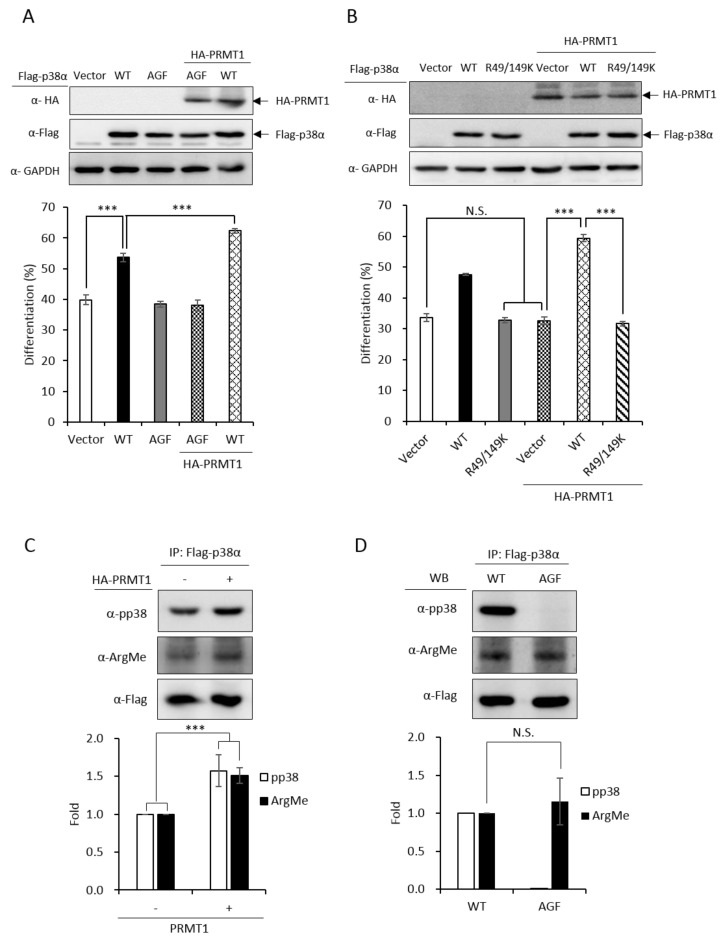
Figure 3.
Promotive effect of PRMT1 on erythroid differentiation is mediated by methylation on R49 and R149 of p38α. The wild-type and AGF (Ala-Gly-Phe) mutant Flag-p38α were expressed in p38α-knockdown cells. The wild-type p38α (WT) promoted differentiation but the phosphorylation activation-deficient AGF mutant was unable to (A). HA-PRMT1 could further promote only in the presence of wild-type p38α but not the p38α AGF mutant (A). In the same p38α KD (knockdown) context, PRMT1 was unable to promote differentiation when R49 and R149 were mutated to K49 and K149 (B). Flag-p38α was expressed in the presence or absence of HA-PRMT1. After AraC stimulation, p38α was immunoprecipitated and examined by Western Blotting using anti-phospho-p38 or anti-methyl arginine antibodies. PRMT1 significantly enhanced p38α phosphorylation and arginine methylation (C). Upon AraC stimulation, both the WT and AGF mutant were methylated to a similar extent, although AGF was deficient in phosphorylation (D). All results shown are representatives of three independent experiments. Erythroid differentiation is presented as the mean ± S.E. of three repeats. The quantification in (C) and (D) was carried out with results from three separate experiments. *** p < 0.005. N.S. means no significance.