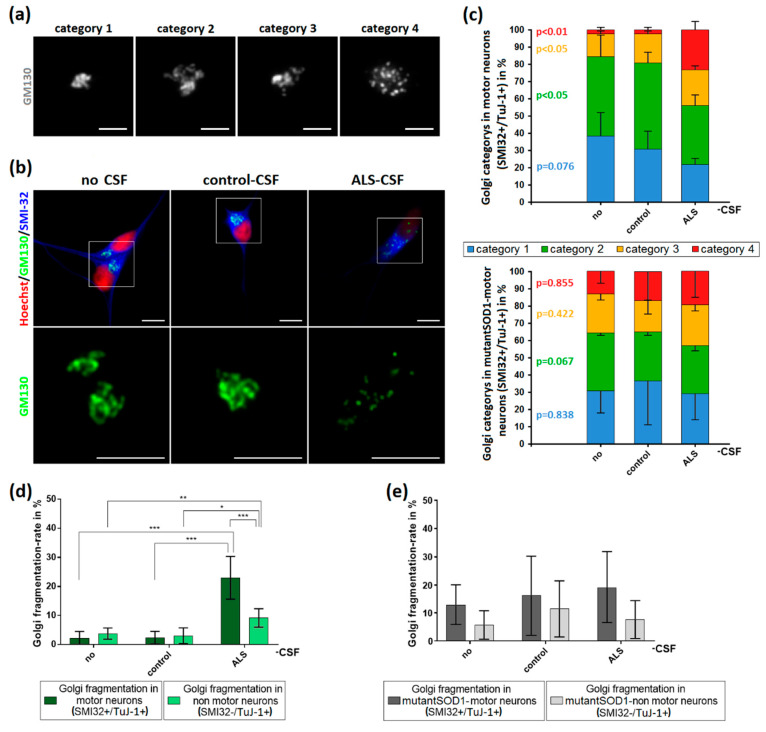
Figure 5.
Induction of Golgi fragmentation predominantly in MNs by the application of ALS-CSF. (a) For analysis, Golgi complexes were categorized depending on their structure. Category 1 was defined as small, compact Golgi apparatus. Single cisterns were difficult to differentiate. In contrast, category 2 included bigger “loosened” Golgi complexes. Cisterns were easier to differentiate. Unlike in the previous ones, category 3 Golgi complexes were not closed compartments anymore. They still formed a cisternal structure, but it was possible to differentiate small cisterns/vesicles not being in contact with the main part of the Golgi. The Golgi apparatus in category 4 was fragmented and only this category accounted for the fragmented ones in our statistics in (d,e). It lost its cohesion and cisternal structure thus became vesicular and the small compartments were dispersed across the cell. (b) The structural Golgi changes in control-human iPSC-induced MNs after ALS-CSF application comprised the loss of cisternal configuration and overall Golgi integrity. (c) The diagrams depict the distribution of the respective Golgi categories in control- (upper diagram) and mutant-SOD1-MNs (lower diagram) ± CSF treatment. (d) The exposition to ALS-CSF caused a significantly higher rate of Golgi fragmentation in WT-human iPSC-induced MNs compared to MNs treated with control-CSF or without CSF. Similar results could be observed comparing SMI32−/TuJ-1+-non MNs treated with ALS-CSF to control-CSF or no CSF-. Of note, SMI32+-MNs exposed to ALS-CSF had a significantly higher Golgi fragmentation rate compared to SMI32−/TuJ-1+-non MNs. (e) The application of ALS-CSF to mutant-SOD1 cells caused no significant rate of Golgi fragmentation neither in SMI32+-MNs nor SMI32-/TuJ-1+-non MNs. Data are depicted as mean ± SEM. */**/** p < 0.05/0.01/0.001 by one-way ANOVA with Tukey post-hoc test. Scale bar = 5 µm.