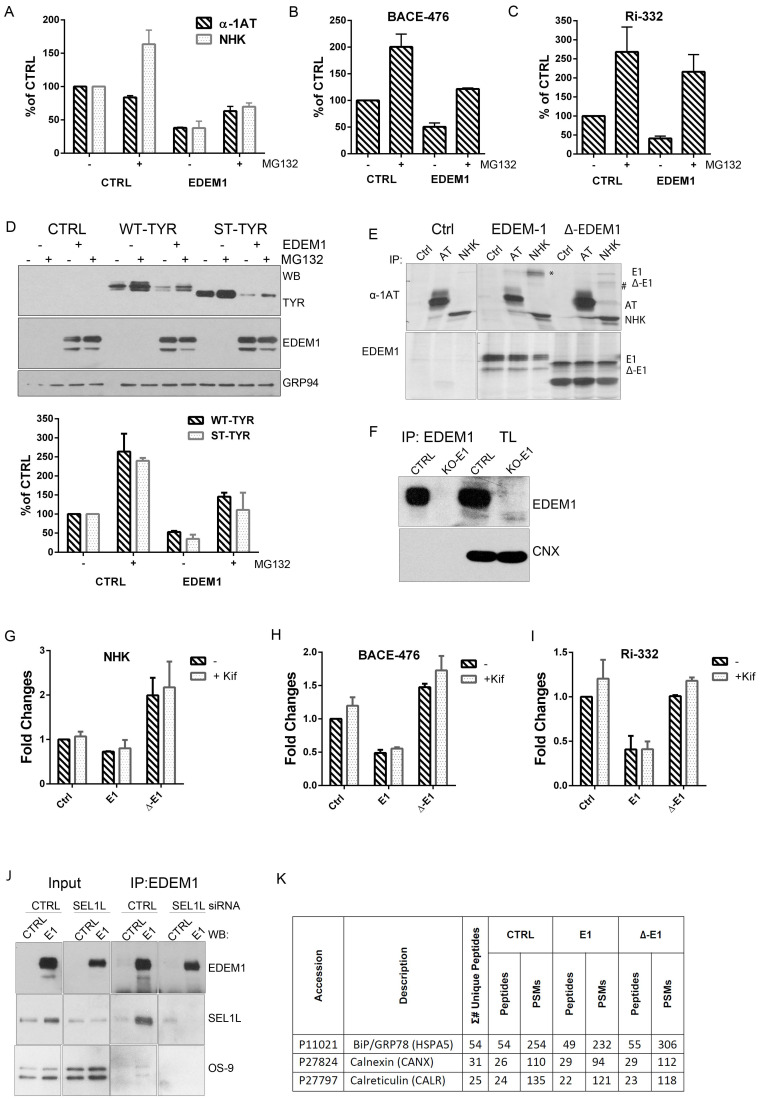
Figure A1.
(A–C) Band densitometry plots of α-1AT and NHK (A), BACE-476 (B), and Ri-332 (C), co-expressed in HEK293T with an empty vector (CTRL) or EDEM1 (EDEM1), treated or not with MG132, as presented in Figure 2A–C (mean n = 2 ± SEM). (D) Cells overexpressing EDEM1 alongside an empty vector (CTRL), WT-Tyr, or its truncated variant ST-Tyr were treated or not with MG132. The pelleted cells were lysed in a Triton-X100-containing buffer, and equal amounts of proteins were separated in reducing conditions by SDS-PAGE. Protein expression was detected by Western blotting using antibodies against α-Tyr, EDEM1, or GRP94 (upper panel); the lower panel depicts band densitometry plots for WT-Tyr and ST-Tyr (mean n = 2 ± SEM). (E) HEK293T co-expressing an empty vector (CTRL), EDEM1 (E1), or Δ-EDEM1 (Δ-E1) with α-AT (AT) and NHK were metabolically labelled with 35S-Met/Cys for 20 min. Harvested cells were lysed in a CHAPS-containing buffer, and cleared lysates were subjected to immunoprecipitation with antibodies for α-AT (upper panel) and EDEM1 (lower panel). The eluted complexes were separated by SDS-PAGE followed by autoradiography. (F) HEK293T (CTRL) and HEK293T-KO for EDEM1 (KO-E1) cells were lysed in a TritonX-100-containing buffer, and cleared lysates were immunoprecipitated with EDEM1 antibodies, captured on Protein A-Sepharose and eluted with a 1× Laemmli buffer. The elutions corresponding to each condition were separated by SDS-PAGE, transferred onto nitrocellulose membrane, and probed for EDEM1 and CNX. (G-I) The band densitometry plots of bands detected for total lysates (TL) of NHK (G), BACE-476 (H), and Ri-332 (I), co-expressed in HEK293T-KO with an empty vector (CTRL), EDEM1 (EDEM1), or ∆-EDEM1 (∆-E1) treated or not with kifunensine (kif), as presented in Figure 2K–M (mean n = 2 ± SEM). (J) HEK293T cells were transfected with siRNA for a non-coding RNA sequence (CTRL) and SEL1L for 72 h. In the last 24 h of transfection, the cells were transfected with a plasmid encoding for EDEM1 (E1) or an empty vector (CTRL). The samples lysed in a CHAPS-containing buffer were immunoprecipitated with antibodies against EDEM1, captured on Protein A-Sepharose, and eluted with a 1× Laemmli buffer; the eluted samples were used for Western blotting, and the membranes were probed with antibodies against EDEM1, SEL1L, and OS-9. (K) Expression of BiP, CNX, and calreticulin for total lysates of HEK293T cells expressing an empty vector, wild type EDEM1, or Δ-EDEM1 analysed by mass spectrometry-based proteomics. The total number of peptides and PSMs identified for each protein is indicated in the figure.