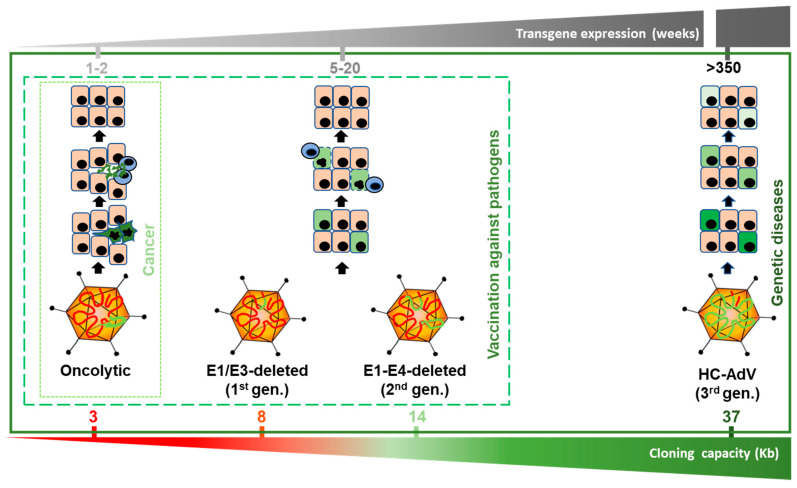
Figure 1.
Versions of adenoviral vectors and potential therapeutic applications. The size range allowed for genome packaging is between 28 and 38 Kb. Oncolytic adenoviruses retain most of the viral genome, including the E1 region, which is required for replication. They can accommodate up to 3 Kb of exogenous DNA if the E3 region is partially deleted. Since they replicate their genomes and cause the lysis of infected cells, transgene expression is very intense but transient. Among replication-deficient vectors, E1/E3-deleted (1st generation) versions can harbor up to 8 Kb. This capacity can be extended up to 14 Kb if the E2 and E4 regions are also deleted (second generation). Although these vectors do not cause direct destruction of infected cells, cytotoxic immune responses against them limit the stability of transgene expression. Apart from vaccination strategies, E1/E3-deleted vectors are still a widely used research tool for in vitro and in vivo gene transfer. High-capacity adenoviral vectors (HC-AdVs) (third generation) only retain short non-coding regions from the AdV genome (ITRs and ψ signal), which leaves a cloning capacity of 37 Kb. The lack of viral gene expression in transduced cells reduces cellular immune responses and allows long-term transgene expression, which decreases slowly as the cells are renewed. HC-AdVs are suitable for all in vivo applications, including gene supplementation and gene correction for monogenic diseases. The indicated duration of transgene expression is based on liver-directed transduction in NHP, but it can be different in other hosts and tissues. In vector genomes, viral DNA is represented in red and exogenous DNA in green (including expression cassettes and/or DNA templates, and stuffer DNA).