Abstract
There has been limited evidence for the association between chronic obstructive pulmonary disease (COPD) and the incidence of lung cancer among never smokers. We aimed to estimate the risk of lung cancer incidence in never smokers with COPD, and to compare it with the risk associated with smoking. This cohort study involved 338 548 subjects, 40 to 84 years of age with no history of lung cancer at baseline, enrolled in the National Health Insurance Service National Sample Cohort. During 2 355 005 person-years of follow-up (median follow-up 7.0 years), 1834 participants developed lung cancer. Compared with never smokers without COPD, the fully-adjusted hazard ratios (95% CI) for lung cancer in never smokers with COPD, ever smokers without COPD, and ever smokers with COPD were 2.67 (2.09 to 3.40), 1.97 (1.75 to 2.21), and 6.19 (5.04 to 7.61), respectively. In this large national cohort study, COPD was also a strong independent risk factor for lung cancer incidence in never smokers, implying that COPD patients are at high risk of lung cancer, irrespective of smoking status.
Keywords: COPD, lung cancer, never smokers
Introduction
Chronic obstructive pulmonary disease (COPD) is associated with increased lung cancer development,1 but this association remains unclear in never smokers. While up to 39% of COPD patients are never smokers,2 there is very limited evidence on the association between COPD and lung cancer incidence in this group, as most studies used cross-sectional or case–control designs and included relatively few never smokers.1 3 In two cohorts, COPD was a significant predictor of incident lung cancer, but the number of incident cases of lung cancer in never smokers with COPD in each cohort was <10.4 5 We used data from a large nationally representative cohort to evaluate the risk of lung cancer incidence associated with COPD by smoking status. Our main objective was to estimate the risk of lung cancer incidence in never smokers with COPD, and to compare this risk to never smokers without COPD, ever smokers without COPD, and ever smokers with COPD.
Methods
Study population and design
We used data from the National Health Insurance Service (NHIS) National Sample Cohort, a population-based retrospective cohort study based on a representative sample of Korean citizens between 1 January 2002 and 31 December 2013.6 We included all men and women, 40 to 84 years of age, who underwent at least one health screening examination provided by the NHIS during the study period (n=370 617). We excluded participants who had cancer (n=8999) before the first screening exam (baseline), or who had missing values for body mass index or smoking status at the baseline exam (n=23 070). The final sample size was 338 548 participants (146 996 men and 191 552 women). The Institutional Review Board of the Samsung Medical Centre approved this study and waived the requirement for informed consent as we used only de-identified data.
Data sources
NHIS claims for inpatient visits, outpatient visits, procedures and prescriptions were coded using the International Classification of Diseases, 10th Revision and the Korean Drug and Anatomical Therapeutic Chemical Codes.7 Lung cancer was defined as the presence of the same C33 or C34 code more than three times within a year or an inpatient hospitalisation with a C33 or C34 code.8 COPD was defined as the presence of J43-J44 (except J43.0) codes and use of COPD medications at least twice within a year.9 Smoking habits were measured by self-administered questionnaires at the baseline screening examination and categorised as never or ever.
Statistical analysis
The study endpoint was lung cancer incidence. Participants were included at the baseline screening examination and followed-up until the development of cancer, death, or the end of the study period (31 December 2013). The study exposure was COPD, considered as a time-varying variable. To account for competing risks due to mortality, we fitted a proportional subdistribution hazards regression model for lung cancer incidence associated with COPD with death as a competing event and age as the time scale. Regression models were adjusted for sex, body mass index and Charlson comorbidity index. We considered a value of p<0.05 as statistically significant. All analyses were performed using STATA version 14 (StataCorp LP, College Station, TX, USA).
Results
Compared with participants without COPD, those who had COPD were older, more likely to be male, smokers, and to have a lower income and more comorbidities (table 1). During 2 355 005 person-years of follow-up (median follow-up 7.0 years), we observed 290 incident cases of lung cancer in participants with COPD (incidence rate 4.9 per 1000 person-years) and 1544 cases in participants without COPD or before the development of COPD (incidence rate 0.7 per 1000 person-years) (table 2)—thus, a total of 1834 participants developed lung cancer.
Table 1.
Characteristics of study participants at the beginning of follow-up (n=338 548)
| Baseline characteristic | Overall (n=338 548) | COPD* | P value | |
| No (n=326 169) | Yes (n=12 379) | |||
| Sex | <0.001 | |||
| Male | 146 996 (43.4) | 140 581 (43.1) | 6415 (51.8) | |
| Female | 191 552 (56.6) | 184 588 (56.9) | 5964 (48.2) | |
| Age (years) | 52.9 (10.5) | 52.5 (10.3) | 62.0 (9.6) | <0.001 |
| <60 | 247 301 (73.1) | 243 538 (74.7) | 3763 (30.4) | |
| 60–69 | 60 595 (17.9) | 55 954 (17.2) | 4641 (37.5) | |
| ≥70 | 30 652 (9.1) | 26 677 (8.2) | 3975 (32.1) | |
| Income percentile | <0.001 | |||
| ≤30th | 72 260 (21.3) | 68 991 (21.2) | 3269 (26.4) | |
| >30th–≤70th | 123 133 (36.4) | 118 860 (36.4) | 4273 (34.5) | |
| >70th | 143 155 (42.3) | 138 318 (42.4) | 4837 (39.1) | |
| Residential area | <0.001 | |||
| Metropolitan | 218 442 (64.5) | 212 255 (65.1) | 6187 (50.0) | |
| Rural | 120 106 (35.5) | 113 914 (34.9) | 6192 (50.0) | |
| Body mass index (kg/m2) | 23.92 (3.13) | 23.93 (3.12) | 23.78 (3.39) | <0.001 |
| Underweight | 9286 (2.7) | 8569 (2.6) | 717 (5.8) | |
| Normal | 126 645 (37.4) | 122 082 (37.4) | 4563 (36.9) | |
| Overweight | 87 561 (25.9) | 84 678 (26.0) | 2883 (23.3) | |
| Obese | 115 056 (34.0) | 110 840 (34.0) | 4216 (34.1) | |
| Smoking status | <0.001 | |||
| Never | 241 633 (71.4) | 233 266 (71.5) | 8367 (67.6) | |
| Past | 21 818 (6.4) | 21 016 (6.4) | 802 (6.5) | |
| Current | 75 097 (22.2) | 71 887 (22.0) | 3210 (25.9) | |
| Charlson comorbidity index | 0 (0–1) | 0 (0–1) | 1 (0–1) | <0.001 |
| 0 | 231 106 (68.3) | 223 581 (68.5) | 7525 (60.8) | |
| 1 | 72 788 (21.5) | 69 896 (21.4) | 2892 (23.4) | |
| ≥2 | 34 654 (10.2) | 32 692 (10.0) | 1962 (15.9) | |
Values in the table are mean (SD), median (IQR) or n (%).
We used χ2 tests, t-tests, or rank-sum tests for comparing patients with and without COPD for categorical and continuous variables, as appropriate.
*COPD patients include those with COPD at baseline and those who developed COPD over follow-up.
COPD, chronic obstructive pulmonary disease.
Table 2.
Hazard ratios (95% confidence intervals) for incident lung cancer associated with COPD (n=338 548)
| Person-years | No. of cases | Incidence rate (per 1000 person-years) |
Subdistribution HR (95% CI) | |
| COPD status | ||||
| None | 2 296 032 | 1544 | 0.7 | Reference |
| COPD | 58 972 | 290 | 4.9 | 3.12 (2.66 to 3.65) |
| COPD and smoking status | ||||
| Never smokers without COPD | 1 670 929 | 783 | 0.5 | Reference |
| Never smokers with COPD | 41 266 | 122 | 3.0 | 2.67 (2.09 to 3.40) |
| Ever smokers without COPD | 625 104 | 761 | 1.2 | 1.97 (1.75 to 2.21) |
| Ever smokers with COPD | 17 705 | 168 | 9.5 | 6.19 (5.04 to 7.61) |
*Subhazard ratios for incident lung cancer were modelled with mortality as a competing risk and adjusted for sex, body mass index (continuous) and Charlson comorbidity index (age as time scale).
COPD, chronic obstructive pulmonary disease.
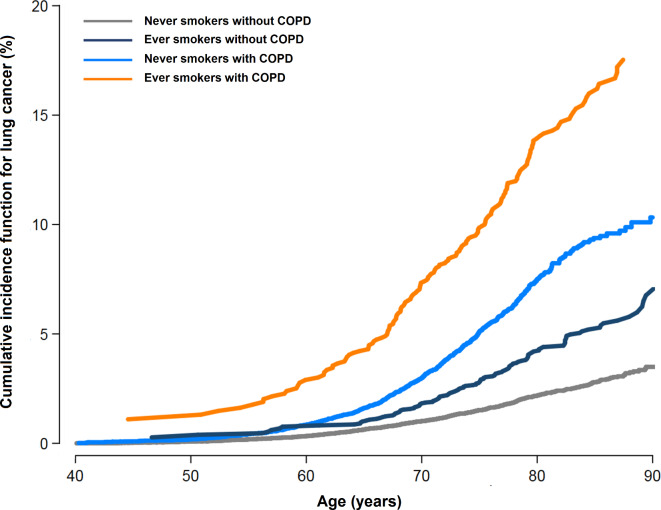
The risk of disease in never smokers with COPD was higher than that in ever smokers without COPD (figure 1). Compared with never smokers without COPD, the fully-adjusted subHR (95% CI) for lung cancer in never smokers with COPD, ever smokers without COPD, and ever smokers with COPD were 2.67 (2.09 to 3.40), 1.97 (1.75 to 2.21), and 6.19 (5.04 to 7.61), respectively (table 2). When we conducted sensitivity analysis in propensity-score matched groups, the results were similar. Among study participants, 70.1% had at least one additional screening during follow-up. Among study participants who were never smokers at baseline, only 2.0% changed to smokers. When we conducted additional analysis using smoking status as the time varying variable, the results were similar (not shown).
Figure 1.
Cumulative incidence function for lung cancer by chronic obstructive pulmonary disease (COPD) and smoking status. COPD was considered as a time-varying exposure. Unexposed person-time was contributed by participants who did not develop COPD and by participants who developed lung cancer before COPD development. Cumulative incidence functions take into account competing risks from all-cause mortality.
Discussion
In this large national cohort study, never smokers with COPD had over 2.6 times the incidence of lung cancer compared with never smokers without COPD. Furthermore, the risk of lung cancer in never smokers with COPD was similar compared with ever smokers without COPD.
Previous studies of COPD and lung cancer incidence were limited by a small number of never smokers with COPD that was insufficient to reliably estimate the association between COPD and lung cancer development in this group.4 5 In another large cohort study, lung cancer mortality was associated with both chronic bronchitis and emphysema, but not with chronic bronchitis alone, in never smokers.10 In this study, the presence of chronic bronchitis and emphysema was established by questionnaire. In our study, based on inpatient, outpatient, procedures, and medication claims, never smokers with COPD had over 2.6 times the incidence of lung cancer compared with never smokers without COPD. Interestingly, the risk of lung cancer development in never smokers with COPD was similar to the risk observed in ever smokers without COPD. Given that poor lung function in COPD is often a barrier to optimal lung cancer treatment due to increased risk of treatment-related morbidities, our study suggests that early detection of lung cancer in COPD patients may reduce the risk of treatment complications.
This study has several limitations. First, COPD severity based on spirometry was not available, thus we could not evaluate the impact of COPD severity on lung cancer incidence. Second, we did not have information on environmental/occupational exposures,11 or severity of emphysema,12 which could be potential confounders for increased lung cancer risk in COPD. Finally, data on clinical outcomes were based on claims data and there might be misclassification of COPD or lung cancer. However, the NHIS routinely audits the claims6 and the data for cancer outcomes are considered highly reliable and have been used in numerous peer-reviewed publications.8 9
In conclusion, COPD was a strong independent risk factor for lung cancer incidence in never smokers. Furthermore, never smokers with COPD had a similar risk of lung cancer compared with ever smokers without COPD. Patients with COPD are at a high risk of lung cancer and future studies should evaluate whether COPD patients are candidates for lung cancer screening, irrespective of smoking status.
Footnotes
HYP and DK contributed equally.
JC and OJK contributed equally.
Contributors: Author contributions: Conception and design: HYP, DK, EG, JC, OJK. Data analysis: DK, EG, JC. Data interpretation and manuscript writing: HYP, DK, SHS, K-HY, CKR, GYS, HK, YMS, EG, JC, OJK. Revision of the manuscript and contribution to intellectual content: HYP, DK, SHS, KHY, CKR, GYS, HK, YMS, EG, JC, OJK.
Funding: This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIP) (no. 2017R1A2B2006435).
Competing interests: None declared.
Patient consent for publication: Not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1. Young RP, Hopkins RJ, Christmas T, et al. . COPD prevalence is increased in lung cancer, independent of age, sex and smoking history. Eur Respir J 2009;34:380–6. 10.1183/09031936.00144208 [DOI] [PubMed] [Google Scholar]
- 2. Zhou Y, Wang C, Yao W, et al. . COPD in Chinese nonsmokers. Eur Respir J 2009;33:509–18. 10.1183/09031936.00084408 [DOI] [PubMed] [Google Scholar]
- 3. Skillrud DM, Offord KP, Miller RD. Higher risk of lung cancer in chronic obstructive pulmonary disease. A prospective, matched, controlled study. Ann Intern Med 1986;105:503–7. 10.7326/0003-4819-105-4-503 [DOI] [PubMed] [Google Scholar]
- 4. Purdue MP, Gold L, Jarvholm B, et al. . Impaired lung function and lung cancer incidence in a cohort of Swedish construction workers. Thorax 2007;62:51–6. 10.1136/thx.2006.064196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mannino DM, Aguayo SM, Petty TL, et al. . Low lung function and incident lung cancer in the United States: data from the first National Health and Nutrition Examination Survey follow-up. Arch Intern Med 2003;163:1475–80. 10.1001/archinte.163.12.1475 [DOI] [PubMed] [Google Scholar]
- 6. Lee J, Lee JS, Park S-H, et al. . Cohort profile: the National Health Insurance Service-National Sample Cohort (NHIS-NSC), South Korea. Int J Epidemiol 2017;46:e15. 10.1093/ije/dyv319 [DOI] [PubMed] [Google Scholar]
- 7. Chang Bae C, Soon Yang K, Jun Young L, et al. . Republic of Korea. Health system review. Health Systems in Transition 2009;11. [Google Scholar]
- 8. Hwangbo Y, Kang D, Kang M, et al. . Incidence of diabetes after cancer development: a Korean national cohort study. JAMA Oncol 2018;4:1099–105. 10.1001/jamaoncol.2018.1684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Park HY, Kang D, Lee H, et al. . Impact of chronic obstructive pulmonary disease on mortality: a large national cohort study. Respirology 2019;46 10.1111/resp.13678 [DOI] [PubMed] [Google Scholar]
- 10. Turner MC, Chen Y, Krewski D, et al. . Chronic obstructive pulmonary disease is associated with lung cancer mortality in a prospective study of never smokers. Am J Respir Crit Care Med 2007;176:285–90. 10.1164/rccm.200612-1792OC [DOI] [PubMed] [Google Scholar]
- 11. Kc R, Shukla SD, Gautam SS, et al. . The role of environmental exposure to non-cigarette smoke in lung disease. Clin Transl Med 2018;7:39 10.1186/s40169-018-0217-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. de Torres JP, Bastarrika G, Wisnivesky JP, et al. . Assessing the relationship between lung cancer risk and emphysema detected on low-dose CT of the chest. Chest 2007;132:1932–8. 10.1378/chest.07-1490 [DOI] [PubMed] [Google Scholar]