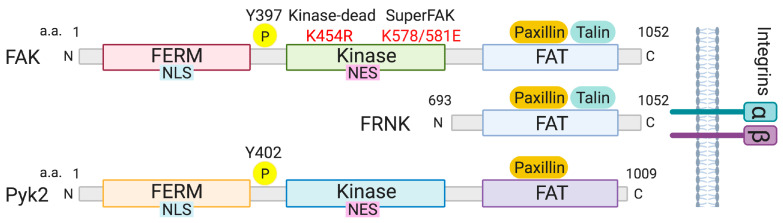
Figure 1.
Structure of FAK, FRNK, and Pyk2. The main domains of FAK, FRNK and Pyk2 are shown. FAK and Pyk2 have three major domains: The N-terminal FERM (band 4.1-ezrin-radixin-moesin) domain, the central kinase domain, and the C-terminal focal adhesion-targeting (FAT) domain. FAK and Pyk2 localize to integrin-containing adhesions via their FAT domains. Upon kinase activation, autophosphorylation at tyrosine (Y) 397 FAK and Y402 Pyk2 provides a binding site for Src-homology 2 (SH2) containing proteins. FAK and Pyk2 shuttle between the nucleus and cytosol through a nuclear localization signal (NLS) and nuclear export signal (NES) in their FERM and kinase domains, respectively. FAK kinase-dead (FAK-KD) is a single nucleotide mutation (lysine 454 to arginine) in the kinase domain resulting in loss of kinase activity. SuperFAK contains two mutations (lysines 578/581 to glutamic acids) that increases catalytic activity of FAK. FRNK (FAK-related nonkinase), which comprises only the C-terminal domain of FAK, is an endogenous inhibitor of FAK. Y397: FAK autophosphorylation site. Y402: Pyk2 autophosphorylation site. a.a.: Amino acids. N: N-terminal. C: C-terminal.