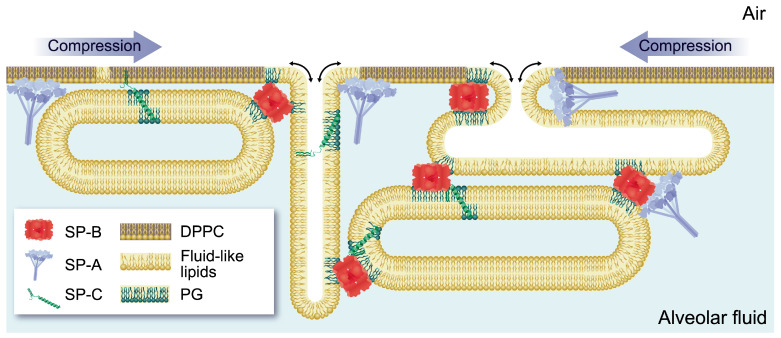
Figure 2.
Lipid–protein interactions in the mechanical stabilization of alveoli during breathing cycles. Surfactant proteins SP-B and SP-C facilitate surfactant dynamics by regulating the mechanical properties of surfactant membranes and films through direct interaction with fluid-like lipids [36], including PG. Binding of SP-B and SP-C to unsaturated and anionic lipids would produce protein partitioning into fluid domains that may contribute to highly curved membranes [37], promoting lipid polymorphism [38,39,40,41,42] and favoring a compression-driven enrichment of the interfacial film in the most surface active component of surfactant, DPPC. On the other hand, by binding to DPPC at solid/fluid phase interfaces, SP-A promotes demixing of surfactant lipids, facilitating the segregation of unsaturated phospholipids at the interface [43] and, thus, modeling the mechanical properties of the surfactant film [44]. In addition, surfactant proteins A, B and C stabilize multilayered interfacial structures and preclude the out-of-plane relaxation of the surfactant film at the end of expiration by promoting membrane-membrane contacts: SP-A (oligomer shown, octadecamer) binds simultaneously to different membranes through its carbohydrate recognition domains [10]; SP-B forms rings (shown as hexamers of dimers) and tubes that connect different bilayers [45,46], and SP-C maintains insertion of palmitoylated cysteins at its N-terminal segment into highly packed liquid-ordered regions of the interfacial film [47,48]. Surfactant membranes may be further stabilized during the breathing cycles by SP-A/SP-B and SP-B/SP-C interactions. Notice that surfactant proteins in the cartoon are not represented at equivalent scale.