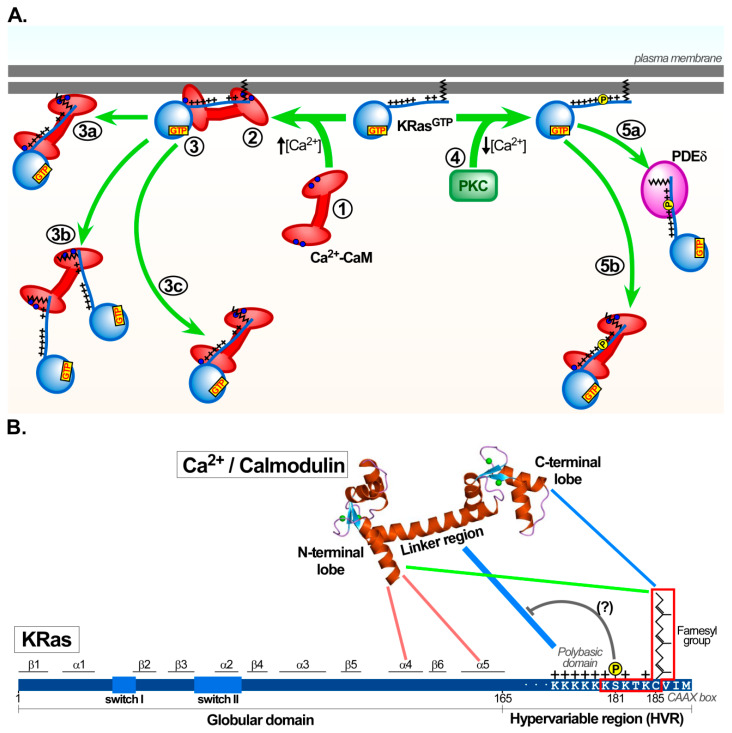
Figure 1.
Calmodulin/KRas interaction motifs and their role in KRas trafficking. The different possibilities how calmodulin may affect KRas trafficking (A) and the protein domains facilitating interaction of calmodulin and KRas are shown (B). (A) Role of calmodulin in KRas trafficking routes. (1) Upon elevation of intracellular [Ca2+] levels, Ca2+ binds and activates calmodulin (Ca2+-CaM), which preferentially interacts with active KRas (KRas-GTP) at the plasma membrane. (2) Initially, the C-terminal lobe and the linker region of Ca2+-CaM interact with the polybasic domain of KRas. The negatively charged Ca2+-CaM linker region is attracted to the polybasic KRas domain through electrostatic coupling. This may be followed by a conformational change in both proteins that permit a secondary interaction between the N-terminal lobe of Ca2+-CaM with the globular domain of KRas, in particular with helices α4 and α5. (3) The majority of Ca2+-CaM/KRas complexes remain associated with the plasma membrane. However, to some extent, the hydrophobic C-terminal lobe of Ca2+-CaM interacts and extracts the KRas farnesyl group from the lipid bilayer. (3a) KRas could remain at the plasma membrane through the interaction of Ca2+-CaM with the acidic membrane leaflet or through interaction with other proteins that are recruited to the plasma membrane, such as phosphoinositide 3-kinase (PI3K). (3b) Alternatively, after binding of the C-terminal lobe of Ca2+-CaM to KRas, followed by a conformational change in Ca2+-CaM, a second KRas protein may bind to the N-terminal lobe of Ca2+-CaM. This would result in a 2:1 Ca2+-CaM/KRas stoichiometry. These KRas proteins could then be removed from the plasma membrane after interaction with Ca2+-CaM. (3c) Ca2+-CaM/KRas complexes with 1:1 stoichiometry may also detach from the plasma membrane. (4) Reduced cytosolic Ca2+ levels lead to the dissociation of the CaM/KRas complex, enabling protein kinase C (PKC) to phosphorylate active KRas at the serine 181 residue (Ser181). (5a) PDEδ or (5b) under certain conditions Ca2+-CaM could cause KRas dissociation from the plasma membrane. The pool of KRas proteins trafficking along the various routes is likely to vary in different cell types and be influenced by changes in the microenvironment and physiological conditions. See text for further details (Section 3.3). (B) Regions involved in KRas and calmodulin interaction. The polybasic domain and the farnesyl group within the hypervariable region (HVR) of KRas are both essential to interact with the linker region and the C-terminal lobe of Ca2+/Calmodulin (blue lines), respectively. The KRas polybasic domain is the initial and primary binding region that interacts with Ca2+/calmodulin (thicker blue line). The minimal Ca2+/calmodulin binding motif of KRas, KSKTKC-farnesyl, is indicated (red square). As part of the KRas/calmodulin interaction, helices α4 and α5 of the globular domain of KRas could also participate interacting with the N-terminal lobe of calmodulin (stoichiometry 1:1; red lines). Alternatively, KRas may first interact with the C-terminal lobe of Ca2+/calmodulin. This could trigger a conformational change of calmodulin to allow interaction of a second KRas protein to the calmodulin N-terminal lobe (stoichiometry 2:1; green line). The position of α-helices, switch I and II within the globular domain (1–165 aas) and the HVR (166–188 aa) of KRas are indicated. The calmodulin N- and the C-terminal lobes, the linker region and the two bound Ca2+ ions in each lobe are also depicted.