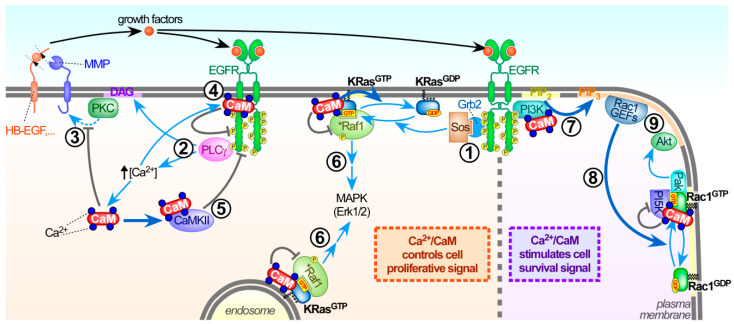
Figure 2.
Model of key signalling events modulated by Ca2+/calmodulin and decisive for cell proliferation and survival. In this scheme, key signalling events regulated by calmodulin (CaM) and critical for cell proliferation (left) and cell survival (right) are highlighted. Left: Several protein–protein interactions of calmodulin enable a negative feedback regulation of the mitogen-activated protein kinase (MAPK) extracellular signal-regulated kinase 1/2 (Erk1/2). This signalling cascade is initially activated by epidermal growth factor receptor (EGFR), and other receptor tyrosine kinases not shown here, at the cell surface and prolonged on endosomes. The overall outcome of these interactions prevents a strong and sustained signal that promotes cell proliferation. Right: Calmodulin can activate phosphoinositide-3 kinase (PI3K) and through protein kinase B (Akt) and Rac1, ensure the generation of signals that stimulate cell survival. Key interactions and signalling events are numbered and underscore the following: (1) Growth factor (e.g., EGF) ¡-induced EGFR activation and autophosphorylation enables the recruitment of adaptors (Grb2) and guanine nucleotide exchange factors (Sos). This is followed by activation of the Ras/Raf1/MAPK signalling pathway. (2) Simultaneously, phospholipase C γ (PLCγ) is recruited to activated EGFR to produce the second messenger diacylglycerol (DAG) and elevate cytosolic Ca2+ levels. (3) The latter binds and activates calmodulin, which disables DAG-dependent and protein kinase C (PKC)-mediated activation of matrix metalloproteases (MMPs). This prevents the shedding and release of membrane-bound growth factor precursors, such as heparin-binding EGF (HB-EGF). This regulatory circuit effectively prevents prolonged EGFR activation in an autocrine manner. (4) In addition, Ca2+/calmodulin binds EGFR and inhibits its tyrosine-kinase activity. (5) Moreover, Ca2+/calmodulin binds Ca2+/calmodulin-dependent kinase II (CaMKII), which has the ability to phosphorylate and inactivate EGFR. These multiple interactions suggest a negative feedback mechanism that allows Ca2+-induced calmodulin activation to trigger downregulation of EGFR signalling. (6) Furthermore, Ca2+/calmodulin may preferentially interact with active KRas (KRas-GTP), which is particularly relevant in settings where KRas is the most prominent Ras family member contributing to MAPK activation, such as in NIH3T3 fibroblasts. In these cells, KRas/calmodulin interaction blocks downstream Raf1 activation. This could possibly be accompanied by the recruitment of GTPase activating proteins (GAPs) that further reduce KRas-GTP levels, thereby advancing to downregulate MAPK signalling and proliferation in these cells. (7) On the other hand, Ca2+/calmodulin together with activated EGFR can stimulate PI3K activity. (8) This increases the recruitment and activity of Rac1 guanine nucleotide exchange factors (Rac1 GEFs) that elevate Rac1-GTP (active) levels. (9) Together with Akt activation, these signalling events are linked to an anti-apoptotic response that triggers cell survival. The GTP/GDP cyles of KRas and Rac1, as well as the membrane location of phosphatidyl-4,5-biphosphate (PIP2) and phosphatidylinositol-3,4,5-triphosphate (PIP3) are indicated. In summary, Ca2+/calmodulin modulates the MAPK pathway driving proliferation and on the other hand, stimulates PI3K activity to induce cell survival. The overall biological outcome probably depends on the signal diversity derived from the extracellular milieu as well as the cell-specific and differential repertoire of calmodulin-responsive players in each cell.