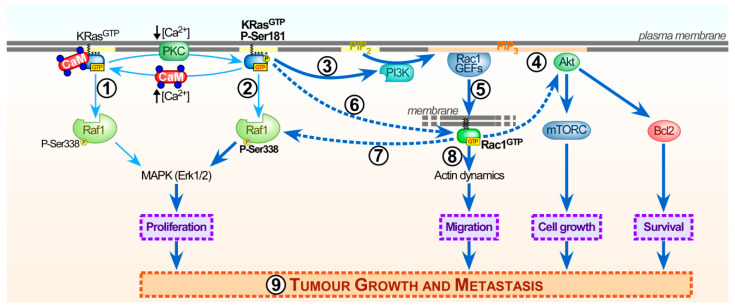
Figure 3.
Model of calmodulin and protein kinase C (PKC) modulating critical features of KRas-driven tumourigenesis. This hypothetical model is based on studies comparing the impact of pharmacological compounds modulating PKC or calmodulin activity in a variety of cell models expressing wild-type or oncogenic and constitutively active KRas (KRasG12V) containing Ser181-phosphomimetic or non-phosphorylatable mutants (see Section 3.4.3 for further details) [16,29,105,121,169,170]. (1) Ca2+/calmodulin binding to the polybasic region (PBR) within the hypervariable region (HVR) of KRas inhibits PKC-mediated Ser181 phosphorylation of KRas (P-Ser181) by sterical hindrance. Complex formation of calmodulin with (active) KRas-GTP may segregate KRas to membrane microdomains where KRas-GTP could be more susceptible to GTPase activating protein (GAP)-mediated KRas inactivation. This ensures KRas inactivation and termination of KRas-GTP-mediated activation of phosphoinositide 3-kinase (PI3K) effector pathways. (2) Low Ca2+ levels disrupt KRas/calmodulin interaction and favor PKC-mediated Ser181 phosphorylation of KRas. (3) This allows a conformational change in P-Ser181 KRas and is followed by its segregation to distinct plasma membrane microdomains or endosomal membranes (omitted in this scheme), where interaction and activation of effectors like PI3K can occur. (4) Ser181 phosphorylation of oncogenic KRas (KRasG12V) triggers PI3K and Akt-dependent anti-apoptotic signals driven by B-cell lymphoma 2 (bcl2) and mammalian target of rapamycin complex (mTORC) that promote survival and cell growth, respectively. (5) PI3K also activates Rac1 guanine nucleotide exchange factors (Rac1 GEFs) that promote activation of Rac1 (Rac1-GTP) on plasma (or endosomal) membranes (dashed lines). The membrane location of phosphatidyl-4,5-biphosphate (PIP2) and phospharidylinositol-3,4,5-triphosphate (PIP3) is indicated. (6) Alternatively, active KRas (KRas-GTP) can directly associate with a Rac1-GEF to activate Rac1. (7) Vice versa, active Rac1-GTP and its effector Pak1 have been suggested to facilitate Ser338 Raf1 phosphorylation and activation, which affects proliferation along the Raf1/mitogen-activated protein kinase (MAPK) pathway. (8) Rac1-GTP drives actin dynamics linked to cell migration. (9) These complex regulatory networks are highlighted by the requirement of Rac1 activity in KRas-driven cancers (see Section 5 for further details). Overall, Ser181 phosphorylation of oncogenic KRas is at the forefront of multiple signalling pathways that are fundamental to cellular events that drive tumour growth and metastasis. This can be counteracted by KRas/calmodulin complex formation, providing a potential tool to reduce signal output of oncogenic KRas.