Abstract
Background
The degree of myocardial injury, as reflected by troponin elevation, and associated outcomes among U.S. hospitalized patients with coronavirus disease-2019 (COVID-19) are unknown.
Objectives
The purpose of this study was to describe the degree of myocardial injury and associated outcomes in a large hospitalized cohort with laboratory-confirmed COVID-19.
Methods
Patients with COVID-19 admitted to 1 of 5 Mount Sinai Health System hospitals in New York City between February 27, 2020, and April 12, 2020, with troponin-I (normal value <0.03 ng/ml) measured within 24 h of admission were included (n = 2,736). Demographics, medical histories, admission laboratory results, and outcomes were captured from the hospitals’ electronic health records.
Results
The median age was 66.4 years, with 59.6% men. Cardiovascular disease (CVD), including coronary artery disease, atrial fibrillation, and heart failure, was more prevalent in patients with higher troponin concentrations, as were hypertension and diabetes. A total of 506 (18.5%) patients died during hospitalization. In all, 985 (36%) patients had elevated troponin concentrations. After adjusting for disease severity and relevant clinical factors, even small amounts of myocardial injury (e.g., troponin I >0.03 to 0.09 ng/ml; n = 455; 16.6%) were significantly associated with death (adjusted hazard ratio: 1.75; 95% CI: 1.37 to 2.24; p < 0.001) while greater amounts (e.g., troponin I >0.09 ng/dl; n = 530; 19.4%) were significantly associated with higher risk (adjusted HR: 3.03; 95% CI: 2.42 to 3.80; p < 0.001).
Conclusions
Myocardial injury is prevalent among patients hospitalized with COVID-19; however, troponin concentrations were generally present at low levels. Patients with CVD are more likely to have myocardial injury than patients without CVD. Troponin elevation among patients hospitalized with COVID-19 is associated with higher risk of mortality.
Key Words: coronavirus, COVID-19, myocardial injury, troponin
Abbreviations and Acronyms: ACE, angiotensin-converting enzyme; AF, atrial fibrillation; ARB, angiotensin II receptor blocker; CAD, coronary artery disease; CKD, chronic kidney disease; COVID-19, coronavirus disease-2019; CVD, cardiovascular disease; DM, diabetes mellitus; EHR, electronic health records; HF, heart failure; HTN, hypertension; MSHS, Mount Sinai Health System; SARS-CoV-2, severe acute respiratory syndrome-coronavirus-2
Central Illustration
Coronavirus disease-2019 (COVID-19), caused by severe acute respiratory syndrome-coronavirus-2 (SARS-CoV-2), is now one of the deadliest pandemics in modern history. The mode of infection of COVID-19 is thought to be direct entry of the SARS-CoV-2 virus into cells via the human angiotensin-converting enzyme 2 (ACE2) receptor, which is expressed predominantly in the lungs but also throughout the cardiovascular system (1). Thus, although the most virulent manifestation of COVID-19 is acute respiratory distress syndrome, reports from Europe and China have also demonstrated cardiac injury reflected through elevated troponin concentrations among infected patients (2, 3, 4, 5). In these limited case series, troponin elevation was more common in patients with pre-existing cardiovascular disease (CVD) and, when present, was associated with higher rates of adverse outcomes in patients hospitalized with COVID-19 (6). However, the observational nature and small sample sizes limit the generalizability of these findings. Additionally, there are no large studies from the United States, the current epicenter of the global pandemic.
As such, major gaps remain in our current understanding of the underlying mechanisms by which SARS-CoV-2 affects the cardiovascular system and how such involvement may alter clinical outcomes: First, the range of troponin elevation across different subpopulations based on history of CVD compared to those without history of CVD is unknown among patients in the United States. Second, whether these troponin elevations represent primary myocardial infarction, supply-demand inequity, or nonischemic myocardial injury remains unclear. Finally, the impact of myocardial injury in the context of COVID-19 infection on outcomes is not well studied. We sought to explore these aims amongst a large cohort of patients hospitalized with COVID-19 in New York City.
Methods
Study population
Patients in this study were drawn from 5 New York City hospitals comprising the Mount Sinai Health System (MSHS): Mount Sinai Hospital, in East Harlem; Mount Sinai West, in Midtown Manhattan; Mount Sinai St. Luke’s, in Harlem; Mount Sinai Queens, in Astoria; and Mount Sinai Brooklyn, in the Midwood neighborhood of Brooklyn. We included all patients admitted to an MSHS hospital with a laboratory-confirmed SARS-CoV-2 infection who were at least 18 years of age and had a troponin measurement within the first 24 h of admission between February 27, 2020, and April 12, 2020. The Mount Sinai Institutional Review Board approved this research under a regulatory protocol allowing for analysis of patient-level COVID-19 data.
Data collection
Data was collected from electronic health records (EHR) from the 5 hospitals. Variables collected included demographics, laboratory measurements, disease diagnoses, comorbidities, procedures, and outcomes (death, intubation, or hospital discharge). Comorbidities were extracted using International Classification of Disease-Revision 9/10 billing codes for atrial fibrillation (AF), asthma, coronary artery disease (CAD), cancer, chronic kidney disease (CKD), chronic obstructive pulmonary disease, diabetes (DM), heart failure (HF), and hypertension (HTN). Troponin I concentrations were assessed via the Abbott Architect method (Abbott, Abbott Park, Illinois), wherein the 99th percentile for a normal population is 0.028 ng/ml. The reference level for normal in MSHS is <0.03 ng/ml. We computed the CURB-65 score for patients at admission to adjust for illness severity on presentation (7). Blood urea nitrogen, respiratory rate, systolic and diastolic blood pressures, and age components of the CURB-65 scoring system were available as structured fields, whereas “confusion” was abstracted using natural language processing of presenting emergency department notes by using the Clinithink engine to encode all identifiable SNOMED concepts and query for positive instances of symptoms related to “Mentally alert” (SNOMED: 248234008) or “Oriented” (SNOMED: 247663003) (8). Patients without either term were classified as having confusion when determining CURB-65 risk strata. Body mass index (BMI) was not available for 219 patients (8.0%). We imputed these BMI values using multiple imputation by chained equations with predictive means matching. Further details of the imputation process are provided in the Supplemental Appendix (Supplemental Figure 1).
Statistical analysis
Descriptive analyses were performed by troponin levels stratified into normal (0.00 to 0.03 ng/ml), mildly elevated (between 1 and 3 times the upper limit of normal, or >0.03 to 0.09 ng/ml), and elevated (more than 3 times the upper limit of normal, or >0.09 ng/ml). Categorical variables were reported as total count and percentage of patients. Continuous nontroponin laboratory values were reported as median and interquartile range. We used troponin measurements within 24 h of admission. If multiple troponin measurements were available within 24 h, the patient’s first measurement was used. We performed analysis of variance to assess for heterogeneity in admission troponin levels across the 5 hospitals included in the present study.
To assess the effects of troponin levels on outcomes, we conducted a survival analysis with the dependent variable of time to mortality, setting time 0 to time of hospital admission. Patients were considered to be right-censored if they were: 1) discharged from the hospital alive; or 2) remained in the hospital at the time of data freeze (Midnight, April 12, 2020). We fit Cox proportional hazards regression models with mortality as the dependent variable, adjusting for age, sex, BMI, race, ethnicity, history of CAD, history of AF, history of HF, history of HTN, history of CKD, history of DM, statin use, ACE inhibitor or angiotensin II receptor blocker (ARB) use, and CURB-65 score at hospital admission. Age was modeled as age at time of admission, while sex, statin use, ACE inhibitor or ARB use, and history of CAD, AF, HTN, CKD, and DM were modeled with binary variables. BMI was modeled as a continuous variable. CURB-65 is an integer score ranging from 0 to 5 representing illness severity. Self-declared race was included in the model with indicator variables corresponding to Caucasian, African American, Asian, Pacific Islander, other, or unknown, with Caucasian as the reference level. Self-declared ethnicity was included in models as Hispanic/Latino, non-Hispanic/Latino, and unknown, with Hispanic/Latino as the reference level. We tested for deviations from the proportional hazards assumption by plotting Martingale residuals from the Cox proportional hazards model versus linearized predictions. The 95% confidence intervals (CIs) from a LOESS best-fit line fit to the Martingale residuals included 0 for all values of the linear predictions, visually indicating that there was not significant deviation from the proportional hazards assumption. We then plotted Kaplan-Meier curves for survival stratified by troponin group.
In addition to the study's primary outcome of mortality, we conducted a secondary analysis examining a composite outcome composed of mortality or intubation with mechanical ventilation. For patients who were intubated and then subsequently died, time to intubation was used. We then conducted a Cox proportional hazards regression analysis controlling for the same covariates as in the primary analysis. Finally, to understand general trends in subsequent troponin values after admission for the subset of patients who had more than 1 troponin measurement, we fit patient-level linear regression models to each patient’s troponin levels as a function of time from initial troponin measurement and split patients into strata based upon the slope of the regression coefficient into those whose troponin levels on average increased or decreased. We then fit Cox proportional hazards regression models to these strata with the dependent variable of time until mortality, again controlling for the same baseline variables. All analyses were conducted in R version 3.6.1 (R Foundation, Vienna, Austria). Survival curve and cumulative incidence visualizations were produced with the survminer R package (7,8).
As a sensitivity analysis, we conducted a complementary analysis where discharge from the hospital was considered to be a competing risk because mortality status could not be assessed after hospital discharge. We used the cmprsk R package for this analysis (9). Hazard ratios for the troponin variables from this analysis were not meaningfully different from our standard survival analysis.
Results
Patient characteristics and troponin levels
During the study period, 3,069 COVID-19 positive patients were hospitalized at 1 of 5 MSHS New York City hospitals. Of these, 2,736 (89.1%) had at least 1 troponin-I measurement within 24 h of admission. The median age was 66.4 years, 40.7% of patients were older than age 70 years, and 40.4% were female. One-quarter of all patients self-identified as African American and 27.6% self-identified as Hispanic or Latino. The mean BMI was 29.8 ± 6.0 kg/m2. A history of CVD, including CAD, AF, or HF was present in 24% of patients, and risk factors only of DM or HTN were present in another 25.8% of the cohort (Table 1). Accordingly, 22% were receiving ACE inhibitors or ARBs, and 36% were receiving statins. CURB-65 scores to represent illness severity displayed increasing trends by troponin strata: patients with troponin levels of 0 to 0.03 ng/ml exhibited a mean CURB-65 score of only 0.90 ± 0.95, whereas patients with troponin levels >0.03 to 0.09 ng/ml were found to have scores of 1.76 ± 1.02 and those with troponin levels >0.09 ng/ml exhibited scores of 2.01 ± 1.05 (p < 0.001) (Table 1 ). Baseline characteristics and outcomes for the 323 (11.8%) patients who did not have troponin measurements assessed within 24 h of admission are shown in Supplemental Table 1. Patients who did not have troponins measured were more likely to be women (55.1% vs. 40.4%; chi-square p < 0.001), were younger (55.0 years vs. 66.4 years; Student's t-test p < 0.001), and had lower rates of medical comorbidities (atrial fibrillation, asthma, CAD, cancer, CKD, chronic obstructive pulmonary disease, diabetes, HF, and HTN; all p < 0.05, 2-proportion Z test with Benjamini-Hochberg Correction for multiple hypothesis testing).
Table 1.
Baseline Characteristics of Admitted Patients, Stratified by Troponin Concentration
| All Patients (n = 2,736) | Troponin I (ng/ml) |
p Value | |||
|---|---|---|---|---|---|
| 0.00–0.03 (n = 1,751) | >0.03-0.09 (n = 455) | >0.09 (n = 530) | |||
| Female | 1,106 (40.4) | 721 (41.2) | 173 (38.0) | 212 (40.0) | 0.463 |
| Race | <0.001 | ||||
| White | 634 (23.2) | 377 (41.2) | 116 (25.5) | 141 (26.6) | |
| African American | 700 (25.6) | 398 (22.7) | 141 (31.0) | 161 (30.4) | |
| Asian | 105 (3.8) | 74 (4.2) | 15 (3.3) | 16 (3.0) | |
| Pacific Islander | 29 (1.1) | 19 (1.1) | 6 (1.3) | 4 (0.8) | |
| Other | 1,157 (42.3) | 806 (46.0) | 163 (35.8) | 188 (35.5) | |
| Unknown race | 111 (4.1) | 77 (4.4) | 14 (3.1) | 20 (3.8) | |
| Ethnicity | 0.001 | ||||
| Hispanic/Latino | 762 (27.9) | 547 (31.2) | 107 (23.5) | 108 (20.4) | |
| Non-Hispanic/Latino | 1,622 (59.3) | 979 (55.9) | 294 (64.6) | 349 (65.8) | |
| Unknown ethnicity | 352 (12.9) | 225 (12.8) | 54 (11.9) | 73 (13.8) | |
| Age, yrs | <0.001 | ||||
| 18–29 | 49 (1.8) | 46 (2.6) | 2 (0.4) | 1 (0.2) | |
| 30–39 | 161 (5.9) | 146 (8.3) | 3 (0.7) | 12 (2.3) | |
| 40–49 | 248 (9.1) | 209 (11.9) | 21 (4.6) | 18 (3.4) | |
| 50–59 | 470 (17.2) | 357 (20.4) | 62 (13.6) | 51 (9.6) | |
| 60–69 | 694 (25.4) | 474 (27.1) | 114 (25.1) | 106 (20.0) | |
| 70–79 | 596 (21.8) | 337 (19.2) | 117 (25.7) | 142 (26.8) | |
| 80–89 | 400 (14.6) | 153 (8.7) | 104 (22.9) | 143 (27.0) | |
| 90–100 | 117 (4.3) | 29 (1.7) | 32 (7.0) | 56 (10.6) | |
| Clinical covariates | |||||
| Body mass index, kg/m2 | 29.8 ± 6.5 | 30.07 ± 6.46 | 29.16 ± 6.44 | 29.08 ± 6.51 | 0.002 |
| ACE inhibitor or ARB use | 601 (22.0) | 332 (19.0) | 118 (25.9) | 151 (28.5) | <0.001 |
| Statin use | 984 (36.0) | 516 (29.5) | 223 (49.0) | 245 (46.2) | <0.001 |
| CURB-65 score | 1.26 ± 1.10 | 0.90 ± 0.95 | 1.76 ± 1.02 | 2.01 ± 1.05 | |
| Comorbidities | |||||
| Atrial fibrillation | 206 (7.5) | 91 (5.2) | 46 (10.1) | 69 (13.0) | <0.001 |
| Asthma | 229 (8.4) | 154 (8.8) | 36 (7.9) | 39 (7.4) | 0.537 |
| Coronary artery disease | 453 (16.6) | 171 (9.8) | 97 (21.3) | 185 (34.9) | <0.001 |
| History of cancer | 195 (7.1) | 123 (7.0) | 38 (8.4) | 34 (6.4) | 0.481 |
| Chronic kidney disease | 273 (10.0) | 90 (5.1) | 66 (14.5) | 117 (22.1) | <0.001 |
| COPD | 158 (5.8) | 70 (4.0) | 39 (8.6) | 49 (9.2) | <0.001 |
| Diabetes | 719 (26.3) | 378 (21.6) | 153 (33.6) | 188 (35.5) | <0.001 |
| Heart failure | 276 (10.1) | 75 (4.3) | 67 (14.7) | 134 (25.3) | <0.001 |
| Hypertension | 1,065 (38.9) | 595 (34.0) | 205 (45.1) | 265 (50.0) | <0.001 |
| Laboratory values | |||||
| Hemoglobin, g/dl | 12.70 (11.30–13.90) | 12.90 (11.70–14.00) | 12.60 (11.20–13.90) | 11.90 (10.20–13.40) | <0.001 |
| Lymphocyte, % | 13.20 (8.12–20.30) | 14.90 (9.10–21.87) | 11.20 (7.10–18.45) | 9.70 (6.20–14.60) | <0.001 |
| D-dimer, μg/ml | 1.43 (0.79–2.75) | 1.17 (0.71–2.15) | 1.65 (1.05–3.21) | 2.54 (1.51–4.93) | <0.001 |
| D-dimer above 1 μg/ml | 1,453 (66.2) | 846 (58.5) | 282 (76.4) | 325 (85.8) | <0.001 |
| C-reactive protein, mg/l | 126.69 (63.71–214.20) | 114.25 (56.61–194.80) | 136.78 (72.30–228.95) | 149.94 (95.09–246.65) | <0.001 |
| Creatine kinase, U/l | 177.50 (83.25–502.50) | 136.00 (72.50–326.75) | 336.00 (120.00–981.50) | 332.00 (155.00–1,015.00) | <0.001 |
| Lactate dehydrogenase, U/l | 441.00 (332.00–592.00) | 425.00 (325.00–551.00) | 456.00 (339.50–616.75) | 520.00 (368.00–753.50) | <0.001 |
| Ferritin, ng/l | 780.50 (376.00–1,899.00) | 724.00 (350.8–1,629.8) | 828.00 (378.00–1,858.50) | 1,093.00 (488.00–2,696.00) | <0.001 |
| Procalcitonin, ng/ml | 0.21 (0.09–0.69) | 0.15 (0.07–0.38) | 0.30 (0.12–0.80) | 0.81 (0.28–2.59) | <0.001 |
| Creatinine, mg/dl | 0.98 (0.75–1.58) | 0.85 (0.70–1.12) | 1.25 (0.90–2.08) | 2.09 (1.24–4.48) | <0.001 |
| Prothrombin time, s | 14.30 (13.50–15.60) | 14.00 (13.30–14.90) | 14.40 (13.80–16.48) | 15.20 (14.10–16.80) | <0.001 |
| Activated partial thromboplastin time, s | 32.90 (29.52–37.90) | 32.30 (29.40–36.80) | 33.00 (29.70–38.98) | 34.40 (30.10–40.70) | 0.002 |
| Albumin, g/dl | 3.00 (2.60–3.30) | 3.00 (2.70–3.30) | 2.90 (2.50–3.20) | 2.90 (2.50–3.20) | <0.001 |
| Bilirubin (total), mg/dl | 0.60 (0.40–0.80) | 0.60 (0.40–0.80) | 0.60 (0.40–0.80) | 0.60 (0.40–0.90) | 0.048 |
| Sodium, mEq/l | 138.00 (135.00–141.00) | 137.00 (135.00–140.00) | 138.00 (135.00–141.00) | 139.00 (136.00–144.00) | <0.001 |
| Tachycardia (heart rate >100 beats/min) | 647 (23.6) | 393 (22.4) | 95 (20.9) | 159 (30.0) | <0.001 |
| Fever (>38.0°C) | 517 (18.9) | 372 (21.3) | 67 (14.8) | 78 (14.8) | <0.001 |
| Hypotension (SBP <100 mm Hg) | 228 (8.3) | 123 (7.0) | 40 (8.8) | 65 (12.3) | 0.001 |
| SBP >160 mm Hg | 227 (8.3) | 104 (5.9) | 53 (11.7) | 70 (13.2) | <0.001 |
Values are n (%), mean ± SD, or median (interquartile range).
ACE = angiotensin-converting enzyme; ARB = angiotensin receptor blocker; COPD = chronic obstructive pulmonary disease; SBP = systolic blood pressure
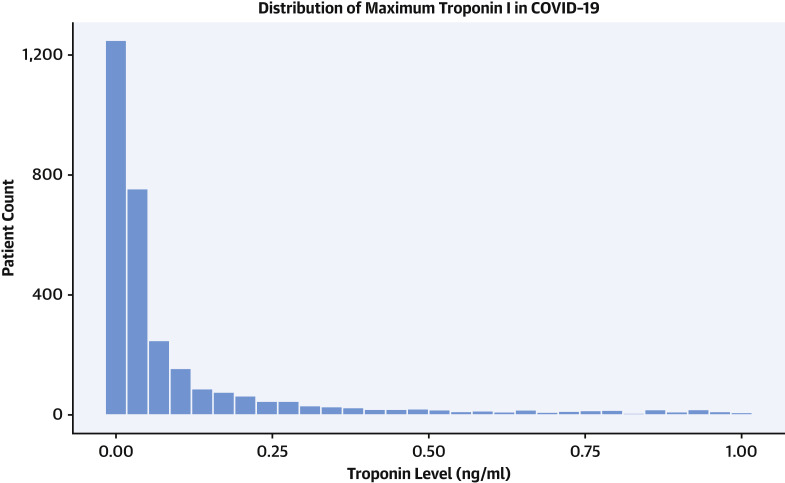
Admission troponin-I concentrations are presented in Figure 1 . Notably, 1,751 (64%) patients had an initial troponin within the normal range. Few patients (86 patients, 3.1%) had an admission troponin over 1 ng/ml within 24 h of admission, while 173 (6.3%) had a troponin elevation over 1 ng/ml at any point during their hospital stay. Patient characteristics as well as admission vital signs and laboratory measurements, stratified by admission troponin-I, are also displayed in Table 1. Troponin elevations were categorized as mildly elevated and elevated as previously defined. Higher troponin concentrations were seen in patients who were over the age of 70 years. Mean presentation troponin levels varied moderately across the 5 hospital sites, ranging from 0.10 ± 0.40 ng/ml to 0.36 ± 2.54 ng/ml (1-way analysis of variance, F = 2.32, df = 5; p = 0.04) (Supplemental Figure 2). However, linear regression revealed that the hospital site explained only 0.4% of the variance in presenting troponin levels (R2 = 0.004).
Figure 1.
Distribution of Maximum In-Hospital Troponin Values for All Patients With Maximum Troponin Values <1.0 ng/ml
Patients with troponin concentrations >1.0 ng/ml are not shown. COVID-19 = coronavirus disease-2019.
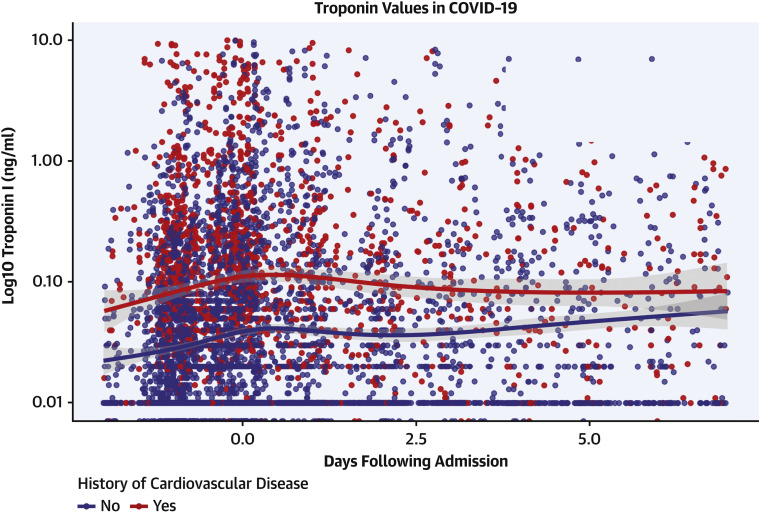
The proportion of patients with CVD (defined here as CAD, AF, or HF) increased with higher troponin concentrations. Specifically, in those patients with more significant myocardial injury (troponin I >0.09 ng/ml), CVD including CAD, AF, and HF, was more prevalent (34.9%, 13.0%, and 25.3%, respectively) compared with patients with mildly elevated troponins (21.3%, 10.1%, and 14.7%, respectively) and those with normal troponins (9.8%, 5.2%, and 4.3%, respectively). Figure 2 plots troponin measurements within 24 h of admission among patients with CVD. Individuals with CVD generally presented with higher initial troponins than those without CVD. Similar trends for myocardial injury were seen in patients with history of HTN, DM, and CKD, but not in those with a history of asthma or cancer. Patient characteristics stratified by history of CVD, risk factors, and no history of either are shown in Table 2 . ACE inhibitor and ARB use as well as statin use were more prevalent amongst increasing troponin strata. They were also more prevalent when stratified by presence of risk factors of CVD and presence of CVD.
Figure 2.
Plot of Longitudinal Troponin Values Over Time, Stratified By History of Cardiovascular Disease or No History of Cardiovascular Disease
Cardiovascular disease includes coronary artery disease, heart failure, and atrial fibrillation. Smoothing lines fit via LOESS regression with shaded areas indicating 95% confidence intervals.
Table 2.
Baseline Characteristics of Admitted Patients Stratified by History of Cardiovascular Disease, Cardiovascular Risk Factors, or Neither
| All Patients (n = 2,736) | Cardiovascular Disease History |
p Value | |||
|---|---|---|---|---|---|
| No Risk Factors (n = 1,374) | Risk Factors (n = 706) | Cardiovascular Disease (n = 656) | |||
| Female | 1,106 (40.4) | 521 (37.9) | 309 (43.8) | 276 (42.1) | 0.022 |
| Age, yrs | 66.40 ± 15.80 | 61.54 ± 16.72 | 65.74 ± 13.75 | 72.98 ± 12.81 | <0.001 |
| Race | <0.001 | ||||
| White | 634 (23.2) | 316 (23.0) | 130 (18.4) | 188 (28.7) | |
| African American | 700 (25.6) | 323 (23.5) | 201 (28.5) | 176 (26.8) | |
| Asian | 105 (3.8) | 48 (3.5) | 28 (4.0) | 29 (4.4) | |
| Pacific Islander | 29 (1.1) | 8 (0.6) | 12 (1.7) | 9 (1.4) | |
| Other | 1,157 (42.3) | 602 (43.8) | 318 (45.0) | 237 (36.1) | |
| Unknown race | 111 (4.1) | 77 (5.6) | 17 (2.4) | 17 (2.6) | |
| Ethnicity | 0.001 | ||||
| Hispanic/Latino | 762 (27.9) | 389 (28.3) | 214 (30.3) | 159 (24.2) | |
| Non-Hispanic/Latino | 1,622 (59.3) | 789 (57.4) | 401 (56.8) | 432 (65.9) | |
| Unknown ethnicity | 352 (12.9) | 196 (14.3) | 91 (12.9) | 65 (9.9) | |
| Clinical covariates | |||||
| Body mass index, kg/m2 | 29.8 ± 6.5 | 29.90 ± 6.51 | 30.40 ± 6.66 | 29.90 ± 6.51 | 0.001 |
| ACE inhibitor or ARB use | 601 (22.0) | 130 (9.5) | 211 (29.9) | 260 (39.6) | <0.001 |
| Statin use | 984 (36.0) | 278 (20.2) | 312 (44.2) | 394 (60.1) | <0.001 |
| CURB-65 score | 1.26 ± 1.10 | 1.00 ± 1.07 | 1.29 ± 1.04 | 1.77 ± 1.03 | <0.001 |
| Laboratory values | |||||
| Hemoglobin, g/dl | 12.70 (11.30–13.90) | 13.00 (11.80–14.0) | 12.40 (11.00–13.80) | 12.30 (10.47–13.50) | <0.001 |
| Lymphocyte, % | 13.20 (8.12–20.30) | 13.10 (8.10–20.10) | 13.90 (8.50–21.20) | 12.90 (7.68–19.72) | 0.133 |
| D-dimer, μg/ml | 1.43 (0.79–2.75) | 1.29 (0.73–2.68) | 1.51 (0.85–2.82) | 1.64 (0.91–2.81) | <0.001 |
| D-dimer above 1 μg/ml | 1,453 (66.2) | 686 (61.9) | 410 (69.6) | 357 (71.8) | <0.001 |
| C-reactive protein, mg/l | 126.69 (63.71–214.20) | 138.71 (72.36–223.43) | 117.88 (61.33–213.63) | 113.34 (53.74–193.88) | <0.001 |
| Creatine kinase, U/l | 177.50 (83.25–502.50) | 218.00 (93.00–487.25) | 163.00 (70.75–549.0) | 131.50 (79.00–425.75) | 0.422 |
| Lactate dehydrogenase, U/l | 441.00 (332.0–592.0) | 456.00 (351.25–611.75) | 436.00 (329.00–574.0) | 409.50 (305.00–553.50) | <0.001 |
| Ferritin, ng/l | 780.50 (376–1,899) | 814.00 (417.0–1,903.0) | 743.00 (356.0–1,850.0) | 765.00 (326.50–1,906.0) | 0.12 |
| Procalcitonin, ng/ml | 0.21 (0.09–0.69) | 0.20 (0.09–0.57) | 0.20 (0.09–0.72) | 0.29 (0.10–0.95) | <0.001 |
| Creatinine, mg/dl | 0.98 (0.75–1.58) | 0.87 (0.70–1.20) | 1.06 (0.78–1.88) | 1.30 (0.90–2.54) | <0.001 |
| Prothrombin time, s | 14.30 (13.50–15.60) | 14.10 (13.50–15.10) | 14.10 (13.30–15.17) | 15.10 (13.90–17.17) | <0.001 |
| Activated partial thromboplastin time, s | 32.90 (29.52–37.90) | 32.30 (29.30–36.20) | 32.00 (29.25–36.55) | 35.70 (31.20–42.27) | 0.002 |
| Albumin, g/dl | 3.00 (2.60–3.30) | 3.00 (2.60–3.30) | 3.00 (2.60–3.30) | 3.00 (2.60–3.30) | 0.703 |
| Bilirubin (total), mg/dl | 0.60 (0.40–0.80) | 0.60 (0.40–0.80) | 0.50 (0.40–0.70) | 0.60 (0.40–0.90) | 0.002 |
| Sodium, mEq/l | 138.00 (135.0–141.0) | 138.00 (135.0–140.0) | 138.00 (135.0–141.0) | 138.00 (135.0–141.0) | 0.057 |
| Tachycardia (heart rate >100 beats/min) | 647 (23.6) | 357 (26.0) | 166 (23.5) | 124 (18.9) | 0.002 |
| Fever (>38.0°C) | 517 (18.9) | 283 (20.6) | 143 (20.3) | 91 (13.9) | 0.001 |
| Hypotension (SBP <100 mm Hg) | 228 (8.3) | 111 (8.1) | 58 (8.2) | 59 (9.0) | 0.762 |
| SBP >160 mm Hg | 227 (8.3) | 84 (6.1) | 68 (9.6) | 75 (11.5) | <0.001 |
Values are n (%), mean ± SD, or median (interquartile range).
Abbreviations as in Table 1.
Acute phase and inflammatory markers were higher among patients with more substantial troponin elevations as well. In particular, median D-dimer, C-reactive protein, lactate dehydrogenase, and procalcitonin were higher in patients with elevated initial troponins (2.54 μg/ml, 149.9 mg/l, 520.0 U/l, and 0.81 ng/ml, respectively) than those with mildly elevated troponins (1.65 μg/ml, 136.78 mg/l, 456.0 U/l, and 0.30 ng/ml, respectively) and those with normal troponins (1.17 μg/ml, 114.25 mg/l, 425.0 U/l, and 0.15 ng/ml, respectively). Patients who had lower hemoglobin, hypotension or hypertension, or tachycardia generally presented with higher troponins than those who did not. In analyzing trends in troponin concentrations over time, we found that 922 patients (33.7%) displayed an increase in troponin concentration after the first 24 h while 811 (29.6%) saw a decrease during hospitalization. The remaining 1,003 (36.7%) did not have further troponin values available for analysis. Of those with increasing troponins over time, 223 (24%) died, compared with 102 (13%) of those with decreasing troponins and 181 (18%) of those with no subsequent troponin measurements. The hazard ratio for mortality in those with an increasing troponin trend compared to those with a decreasing troponin trend was 2.13 (95% CI: 1.68 to 2.70; p < 0.001) after adjusting for age, sex, race, ethnicity, BMI, CAD, DM, HF, HTN, AF, atrial chronic kidney disease, CURB-65 score, ACE-inhibitor or ARB use, and statin use (Supplemental Figure 3).
Outcomes
Mortality
Of 2,736 COVID-19 patients included in our study, 506 (18.5%) died, 1,132 (41.4%) were discharged, and 1,098 (40.1%) remained hospitalized at the time of data freeze for this report. The median length of stay was 5.75 days (Q1 to Q3: 3.36 to 9.56 days). In a Cox proportional hazards regression model, increased age, increased BMI, and higher illness severity (as indicated by higher CURB-65 scores) were associated with increased risk of death, whereas sex, race/ethnicity, and risk factors for CVD and CVD (CAD, AF, HF) were not (Table 3 ). Statin use but not ACE inhibitor or ARB use was associated with improved survival (hazard ratio [HR]: 0.57; 95% CI: 0.47 to 0.69).
Table 3.
Results From Cox Proportional Hazards Regression Analysis for Mortality as a Function of Troponin Strata, Demographics, Race, Ethnicity, Comorbidities, and Clinical Variables Including BMI, CURB-65 Score, ACE inhibitor/ARB Use, and Statin Use
| Coefficient | HR | 95% Confidence Interval | p Value |
|---|---|---|---|
| Troponin strata | |||
| >0.03–0.09 ng/ml | 1.75 | (1.37–2.24) | <0.001 |
| >0.09 ng/ml | 3.03 | (2.42–3.80) | <0.001 |
| Demographics | |||
| Female | 0.85 | (0.71–1.03) | 0.093 |
| Age, yrs | 1.04 | (1.03–1.04) | <0.001 |
| Race | |||
| African American | 0.89 | (0.70–1.14) | 0.371 |
| Asian | 0.95 | (0.56–1.61) | 0.862 |
| Pacific Islander | 1.21 | (0.49–3.00) | 0.681 |
| Other | 1.11 | (0.85–1.44) | 0.451 |
| Unknown | 1.24 | (0.77–2.00) | 0.378 |
| Ethnicity | |||
| Non-Hispanic/Latino | 1.11 | (0.84–1.46) | 0.479 |
| Unknown | 1.39 | (1.01–1.92) | 0.045 |
| Comorbidities | |||
| Coronary artery disease | 1.08 | (0.85–1.37) | 0.535 |
| Diabetes | 1.01 | (0.80–1.27) | 0.947 |
| Heart failure | 1.03 | (0.77–1.37) | 0.867 |
| Hypertension | 0.99 | (0.79–1.23) | 0.905 |
| Atrial fibrillation | 1.08 | (0.81–1.44) | 0.586 |
| Chronic kidney disease | 1.02 | (0.76–1.36) | 0.911 |
| Clinical variables | |||
| BMI, kg/m2 | 1.02 | (1.01–1.03) | 0.007 |
| CURB-65 score | 1.23 | (1.11–1.36) | <0.001 |
| ACE inhibitor or ARB use | 1.05 | (0.85–1.31) | 0.637 |
| Statin use | 0.57 | (0.47–0.69) | <0.001 |
BMI = body mass index; HR = hazard ratio; other abbreviations as in Table 1.
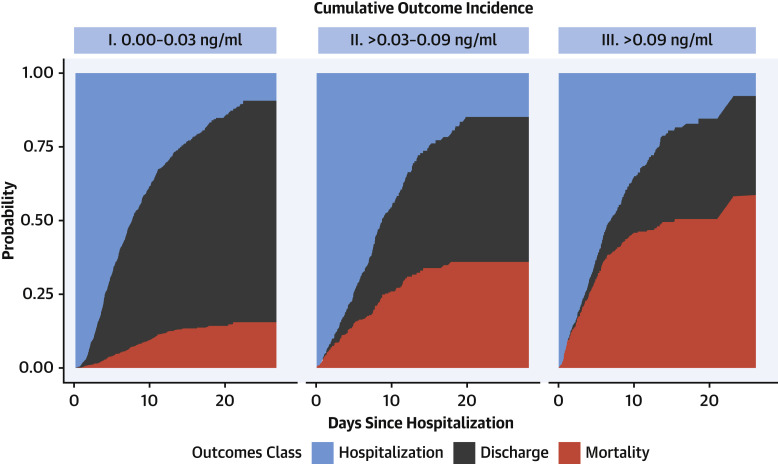
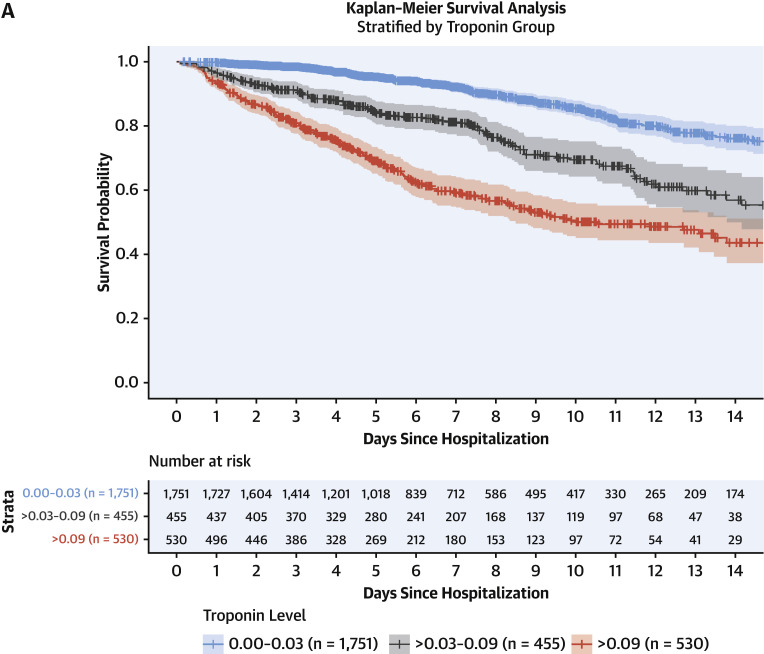
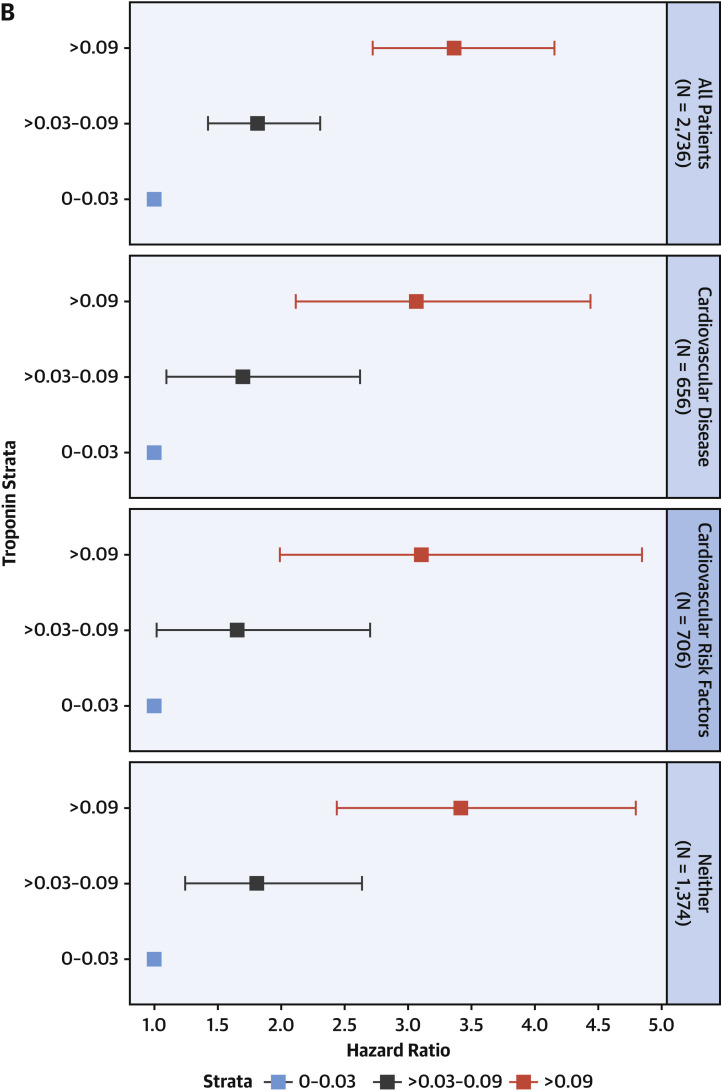
Figure 3 presents cumulative incidence plots displaying probability for 3 possible outcomes (mortality, discharge from hospital, or continued hospitalization) over time. Milder forms of myocardial injury (e.g., troponin concentration >0.03 to 0.09 ng/ml) were associated with less frequent discharge and higher risk of death than troponin levels in the reference range after adjustment for clinically relevant covariates (adjusted HR: 1.75; 95% CI: 1.37 to 2.24) (Figure 4A ). Troponin concentrations over 0.09 ng/dl were associated with more pronounced risk of death (adjusted HR: 3.03; 95% CI: 2.42 to 3.80) after adjustment. This risk was consistent across patients stratified by history of CVD, CVD risk factors such as DM or HTN only, and neither CVD nor risk factors. (Figure 4B). A sensitivity analysis using a competing-risks framework demonstrated similar adjusted hazards ratios for risk of death: HR: 2.02 (95% CI: 1.58 to 2.60) for troponin concentrations >0.03 to 0.09 ng/ml, and HR: 3.52 (95% CI: 2.79 to 4.45) for troponin concentrations >0.09 ng/ml (Supplemental Table 2).
Figure 3.
Cumulative Incidence Plots Displaying Probability for 3 Possible Outcomes (Mortality, Discharge From Hospital, or Continued Hospitalization) Over Time
Cumulative incidence plots displaying probability for 3 possible outcomes (mortality, discharge from hospital, or continued hospitalization) over time.
Figure 4.
Survival Past Hospital Admission, Stratified by Troponin Grouping
(A) Patients were considered to be right-censored if they were discharged alive from the hospital or were still hospitalized at the time of data freeze (April 12, 2020). Survival times were significantly different between groups (p < 0.001). (B) Hazard ratios and 95% confidence intervals calculated by Cox proportional hazards regression models for mortality stratified by comorbidities. Patients with cardiovascular disease had comorbidities of coronary artery disease, heart failure, or atrial fibrillation. Patients with cardiovascular risk factors had comorbidities of diabetes mellitus or hypertension, but not cardiovascular disease.
Composite outcome of mortality or mechanical ventilation
Altogether, 813 of 2,746 patients (29.6%) either died or underwent intubation by the end of the period of observation, compared with the 506 patients who died in the primary analysis (18.4%). In a Cox proportional hazards regression analysis controlling for the same covariates as in the primary analysis, troponin elevation remained a significant predictor of outcomes. We observed an HR of 1.75 (95% CI: 1.44 to 2.13; p < 0.001) for troponin concentrations >0.03 to 0.09 ng/ml, and an HR of 2.97 (95% CI: 2.47 to 3.56) for troponin concentrations >0.09 ng/ml. A Kaplan-Meier plot demonstrating the trends in composite outcome by troponin strata is provided in Supplemental Figure 4.
Discussion
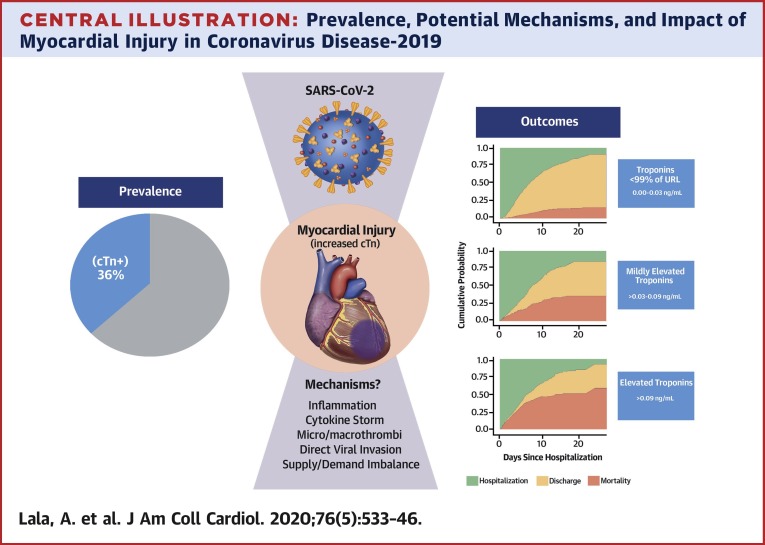
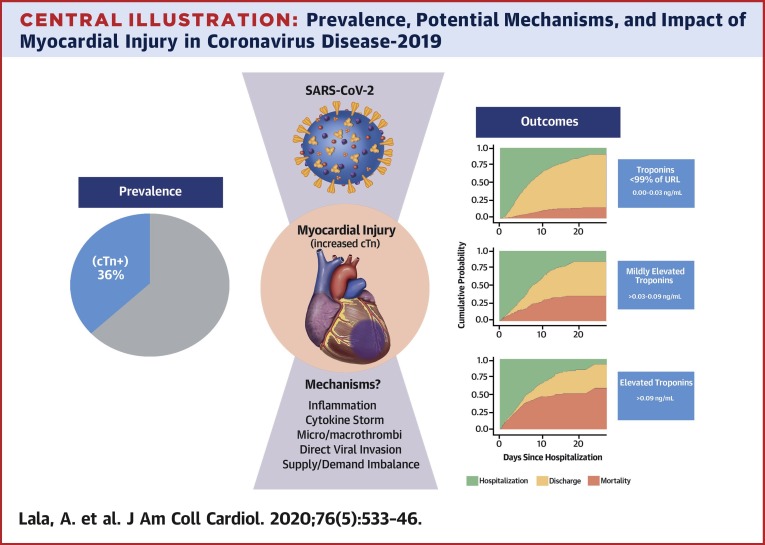
Although pulmonary manifestations are its most common consequence, COVID-19 causes systemic inflammation with varying presentations of cardiac involvement as well (10). In this multihospital retrospective cohort study of nearly 3,000 patients, we demonstrate the following observations: 1) myocardial injury is common among patients hospitalized with COVID-19 but is more often mild, associated with low-level elevation in troponin concentration; 2) more significant myocardial injury may be associated with more than a tripling in risk of mortality; and 3) COVID-19 patients with a history of CVD are more likely to experience myocardial injury than patients without CVD but without obvious corroborating evidence for primary acute myocardial infarction (Central Illustration ).
Central Illustration.
Prevalence, Potential Mechanisms, and Impact of Myocardial Injury in Coronavirus Disease-2019
Myocardial injury reflected by troponin concentrations above the upper reference limit (URL) of 0.03 ng/ml was present in 36% of patients hospitalized with coronavirus disease-2019 (COVID-19). Troponin levels among patients hospitalized with COVID-19 were generally <1.0 ng/ml. Even small amounts of myocardial injury (e.g., troponin I >0.03 to 0.09 ng/ml, n = 455 [16.6%]) were associated with death (adjusted HR: 1.75; 95% confidence interval: 1.37 to 2.24) while greater amounts (e.g., troponin I >0.09 ng/dl, n = 530 [19.4%]) were associated with more pronounced risk for death (adjusted HR: 1.77; 95% confidence interval: 1.39 to 2.26; p < 0.001). Troponin elevation in the setting of acute COVID-19 may primarily reflect nonischemic or secondary myocardial injury, but the true mechanism remains unknown. SARS-CoV-2 = severe acute respiratory syndrome-coronavirus-2.
Although troponin elevation above the 99th percentile of the upper reference limit (URL) is considered the central marker of “myocardial injury” (11), underlying pathophysiological mechanisms must be elucidated according to clinical circumstances. Myocardial injury is best recognized in the context of ischemia; however, several nonischemic-mediated mechanisms, which include apoptosis, myocardial strain, myocyte necrosis, and increased cell membrane permeability-mediated exocytotic release of troponin, may contribute to such injury (12,13). According to the Fourth Universal Definition of Myocardial Infarction, very few patients met strict criteria for acute myocardial infarction. Although some patients in this cohort certainly experienced ischemic myocardial damage from either type 1 or 2 myocardial infarction, it is possible that a majority of injury observed was mediated through a noncoronary mechanism. Challenges exist, however, regarding understanding underlying etiology.
Despite several reports of COVID-19 associated myocarditis, to date, no case has demonstrated COVID-19 genome in cardiac tissue on biopsy or autopsy accompanied by troponin elevation consistent with criteria used to diagnose myocarditis (3, 4, 5,14, 15, 16). Other postulated mechanisms by which COVID-19 leads to cardiovascular morbidity include direct myocardial injury as a result of the inflammatory cascade or cytokine release, microvascular damage due to disseminated intravascular coagulation and thrombosis, direct entry of SARS-CoV-2 into myocardial cells via binding to ACE2 receptors, hypoxemia combined with increased metabolic demands of acute illness leading to myocardial injury akin to type 2 myocardial infarction, and finally, acute coronary syndrome from acute inflammation-triggered destabilization of atheromas (17, 18, 19).
In a recent case series of 18 patients with COVID-19 infection and ST-segment elevation on electrocardiogram, 10 were deemed to have noncoronary myocardial injury by virtue of nonobstructive disease on coronary angiography and/or normal wall motion on echocardiography (20). Despite lower troponin concentrations in this group, 9 died as opposed to 4 of 8 in the ST-segment elevation MI group, which may suggest higher mortality associated with nonischemic mediated myocardial injury in the setting of COVID-19; however, more data are needed.
We demonstrate that myocardial injury was prevalent among a large hospitalized cohort in the United States, occurring in 36%. Evidence for myocardial injury was more frequent in our cohort compared with recent reports from China (2,21, 22, 23, 24). These prior studies included between 41 and 416 patients and noted prevalence of myocardial injury ranging from 7% to 28%. Similar to these smaller reports, we also noted that patients with myocardial injury tended to be older and have a history of CVD. We also noted lower hemoglobin values, higher inflammatory markers, and more frequent rates of tachycardia or hypotension/hypertension.
Because SARS-CoV-2 enters cells via binding to the ACE2 receptor, previous concerns existed as to increased risk of adverse outcomes conferred by ACE inhibitors or ARBs; these worries have been somewhat dissipated in light of recent studies showing no increased risk associated with the use of these drugs (25,26). We demonstrate a protective association with statin use but no association with ACE inhibitors or ARBs, consistent with a simultaneous report by Reynolds et al. (25). The benefit of statins in the setting of myocardial injury is well established (27, 28, 29), yet whether statins confer an anti-inflammatory effect or allow for amelioration of endothelial dysfunction in COVID-19 have not been elucidated. It is also possible that statin use in-hospital is confounded by physicians’ treatment priorities, as statins may simply be discontinued for patients who are intubated or otherwise became critically ill. Given the impressive effect size we observed in this study (HR: 0.57), it may be that at least some component of statins’ observed efficacy is due to this confounding phenomenon. Interestingly, higher BMI was associated with increased mortality in the setting of COVID-19 among our cohort, consistent with a prior report (30). This may be a reflection of the high prevalence of DM and concomitant metabolic syndrome in our population, although BMI did not differ by troponin strata or the presence of risk factors for CVD or CVD. Data on insulin resistance and cholesterol were not available in our cohort.
Although present as low-level concentrations, troponin elevation to >3 times the URL was associated with a 3-fold increased risk of mortality despite adjustment for clinically relevant factors. This finding is in keeping with a report from Wuhan, China, of 416 patients by Shi et al. (2), which demonstrated an HR of 3.41 (95% CI: 1.62 to 7.16) for death in patients with myocardial injury compared with patients without. Guo et al. (21) reported similar findings among 187 patients also in Wuhan, China, but emphasized that although myocardial injury was more prevalent in patients with history of CVD, outcomes were more favorable in patients with CVD and no myocardial injury as compared with individuals with myocardial injury and no history of CVD. We similarly show that myocardial injury, when present, regardless of history of CVD or risk factors, was associated with worse outcomes inclusive of mechanical intubation or mortality.
Study limitations
First, there are limitations inherent to the use of EHR for patient-level data in such a large sample size not explicitly verified by manual chart review. For example, sample size did not permit manual review of electrocardiogram findings to correlate with troponin elevations. Despite these limitations, the use of EHR enabled timely analysis and rapid dissemination of crucial information in a large patient cohort at the epicenter of the global pandemic. Second, some patients included had not completed their hospital course at the time of data freeze. We accounted for this by conducting a secondary, complementary survival analysis where hospital discharge was treated as a competing risk as outlined in the Methods section. Results from our competing risks analysis were not meaningfully different from a standard survival analysis where discharged patients were simply considered to be right-censored. Use of anticoagulation and antiviral therapy was not included in part due to patient participation in clinical trials leading to incomplete data. Further, natriuretic peptide levels were not available for more than two-thirds of the study cohort within 24 h of admission, and therefore, patterns in the context of myocardial injury could not be described. Outcomes analyses were focused upon troponin measurements made at hospital admission and less upon serial troponin measurements obtained over the course of each patient’s hospital stay, although we also demonstrate that trends in troponin levels over time are associated with mortality. Troponin concentrations were not available in 323 patients who had fewer comorbidities than the 2,736 patients for whom troponin tests were ordered, and as such may have impacted our results. Finally, it was not possible to ascertain mechanisms of death including cardiovascular and noncardiovascular causes.
Conclusions
Myocardial injury is prevalent, generally at low levels, among patients with acute COVID-19 and is associated with worse outcomes. History of CVD was associated with myocardial injury in the setting of COVID-19 infection. These results suggest abnormal troponin concentrations on admission may be helpful with regard to triage decision-making. However, whether treatment strategies based on troponin concentrations would be expected to improve outcomes remains a testable hypothesis.
Perspectives.
COMPETENCY IN MEDICAL KNOWLEDGE: Myocardial injury, reflected by troponin elevation, is common among patients hospitalized with COVID-19, particularly among those with a history of cardiovascular disease, and is associated with a high risk of mortality.
TRANSLATIONAL OUTLOOK: Further research is needed to elucidate the mechanisms responsible for myocardial injury in patients with COVID-19 and compare clinical outcomes associated with ischemic versus nonischemic types.
Acknowledgment
The authors thank Sayan Manna for his work in preparation of this manuscript.
Footnotes
This work was supported by U54 TR001433-05, National Center for Advancing Translational Sciences, National Institutes of Health. Dr. Januzzi is a Trustee of the American College of Cardiology; has received grant support from Novartis Pharmaceuticals and Abbott Diagnostics; has received consulting income from Abbott, Janssen, Novartis, MyoKardia, and Roche Diagnostics; and has participated in clinical endpoint committees/data safety monitoring boards for Abbott, AbbVie, Amgen, CVRx, Janssen, and Takeda. Dr. Pinney has received consulting fees from Abbott, CareDx, Medtronic, and Procyrion. Dr. Fayad has received consulting fees from Alexion and GlaxoSmithKline; has received research funding from Daiichi-Sankyo, Amgen, Bristol-Myers Squibb, and Siemens Healthineers; has received financial compensation as a board member and advisor to Trained Therapeutix Discovery; and owns equity in Trained Therapeutix Discovery as cofounder. Dr. Nadkarni has received consulting fees from AstraZeneca, Reata, BioVie, and GLG Consulting; has received financial compensation as a scientific board member and advisor to RenalytixAI; and owns equity in RenalytixAI and Pensieve Health as a cofounder. Dr. Johnson has received personal fees from Tempus Labs; and holds equity in Tempus Labs and Oova. Dr. Van Vleck has received financial compensation as a consultant for Clinithink, Ltd., the developer of the natural language processing software utilized in this study; and owns equity in Clinithink, a privately traded company. Dr. Lala has received speaker honoraria from Zoll. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose. Patrick O'Gara, MD, served as Guest Associate Editor for this paper. P.K. Shah, MD, served as Guest Editor-in-Chief for this paper.
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the JACCauthor instructions page.
Appendix
For supplemental figures and tables, please see the online version of this paper.
Appendix
References
- 1.South A.M., Diz D.I., Chappell M.C. COVID-19, ACE2, and the cardiovascular consequences. Am J Physiol Heart Circ Physiol. 2020;318:H1084–H1090. doi: 10.1152/ajpheart.00217.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shi S., Qin M., Shen B. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiology. 2020 Mar 25 doi: 10.1001/jamacardio.2020.0950. [E-pub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Inciardi R.M., Lupi L., Zaccone G. Cardiac involvement in a patient with coronavirus disease 2019 (COVID-19) JAMA Cardiology. 2020 Mar 27 doi: 10.1001/jamacardio.2020.1096. [E-pub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zeng J.H., Liu Y.X., Yuan J. First case of COVID-19 complicated with fulminant myocarditis: a case report and insights. Infection. 2020 Apr 10 doi: 10.1007/s15010-020-01424-5. [E-pub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hu H., Ma F., Wei X., Fang Y. Coronavirus fulminant myocarditis saved with glucocorticoid and human immunoglobulin. Eur Heart J. 2020 Mar 16 doi: 10.1093/eurheartj/ehaa190. [E-pub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lippi G., Lavie C.J., Sanchis-Gomar F. Cardiac troponin I in patients with coronavirus disease 2019 (COVID-19): evidence from a meta-analysis. Prog Cardiovasc Dis. 2020 Mar 10 doi: 10.1016/j.pcad.2020.03.001. [E-pub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.R Core Team R: a language and environment for statistical computing. R Foundation for Statistical Computing. 2017. https://www.R-project.org/ Available at:
- 8.Kassambara A., Kosinski M., Biecek P. Survminer: drawing survival curves using “ggplot2.” 2019. https://cran.r-project.org/package=survminer Available at:
- 9.Gray B. cmprsk: subdistribution analysis of competing risks. 2019. https://cran.r-project.org/package=cmprsk Available at:
- 10.Fried J.A., Ramasubbu K., Bhatt R. The variety of cardiovascular presentations of COVID-19. Circulation. 2020;141:1930–1936. doi: 10.1161/CIRCULATIONAHA.120.047164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thygesen K., Alpert J.S., Jaffe A.S. Fourth universal definition of myocardial infarction (2018) J Am Coll Cardiol. 2018;72:2231–2264. doi: 10.1016/j.jacc.2018.08.1038. [DOI] [PubMed] [Google Scholar]
- 12.Park K.C., Gaze D.C., Collinson P.O., Marber M.S. Cardiac troponins: from myocardial infarction to chronic disease. Cardiovasc Res. 2017;113:1708–1718. doi: 10.1093/cvr/cvx183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Januzzi J.L., Jr., McCarthy C.P. Trivializing an Elevated Troponin: Adding Insult to Injury? J Am Coll Cardiol. 2019;73:10–12. doi: 10.1016/j.jacc.2018.10.042. [DOI] [PubMed] [Google Scholar]
- 14.Caforio A.L., Pankuweit S., Arbustini E. Current state of knowledge on aetiology, diagnosis, management, and therapy of myocarditis: a position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur Heart J. 2013;34:2636–2648. doi: 10.1093/eurheartj/eht210. 2648a–d. [DOI] [PubMed] [Google Scholar]
- 15.Barton L.M., Duval E.J., Stroberg E., Ghosh S., Mukhopadhyay S. COVID-19 autopsies, Oklahoma, USA. Am J Clin Pathol. 2020;153:725–733. doi: 10.1093/ajcp/aqaa062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tavazzi G., Pellegrini C., Maurelli M. Myocardial localization of coronavirus in COVID-19 cardiogenic shock. Eur J Heart Fail. 2020;22:911–915. doi: 10.1002/ejhf.1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Clerkin K.J., Fried J.A., Raikhelkar J. Coronavirus disease 2019 (COVID-19) and cardiovascular disease. Circulation. 2020;141:1648–1655. doi: 10.1161/CIRCULATIONAHA.120.046941. [DOI] [PubMed] [Google Scholar]
- 18.Patel A.B., Verma A. COVID-19 and angiotensin-converting enzyme inhibitors and angiotensin receptor blockers: what is the evidence? JAMA. 2020 Mar 24 doi: 10.1001/jama.2020.4812. [E-pub ahead of print] [DOI] [PubMed] [Google Scholar]
- 19.Madjid M., Safavi-Naeini P., Solomon S.D., Vardeny O. Potential effects of coronaviruses on the cardiovascular system: a review. JAMA Cardiology. 2020 Mar 27 doi: 10.1001/jamacardio.2020.1286. [E-pub ahead of print] [DOI] [PubMed] [Google Scholar]
- 20.Bangalore S., Sharma A., Slotwiner A. ST-segment elevation in patients with Covid-19—a case series. N Engl J Med. 2020;382:2478–2480. doi: 10.1056/NEJMc2009020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guo T., Fan Y., Chen M. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19) JAMA Cardiology. 2020 Mar 27 doi: 10.1001/jamacardio.2020.1017. [E-pub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhou F., Yu T., Du R. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen T., Wu D., Chen H. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ. 2020;368:m1091. doi: 10.1136/bmj.m1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang C., Wang Y., Li X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reynolds H.R., Adhikari S., Pulgarin C. Renin–angiotensin–aldosterone system inhibitors and risk of Covid-19. N Engl J Med. 2020 May 8 doi: 10.1056/NEJMoa2008975. [E-pub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mancia G., Rea F., Ludergnani M., Apolone G., Corrao G. Renin–angiotensin–aldosterone system blockers and the risk of Covid-19. N Engl J Med. 2020;382:2431–2440. doi: 10.1056/NEJMoa2006923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fang S.-Y., Roan J.-N., Luo C.-Y., Tsai Y.-C., Lam C.-F. Pleiotropic vascular protective effects of statins in perioperative medicine. Acta Anaesthesiol Taiwan. 2013;51:120–126. doi: 10.1016/j.aat.2013.08.003. [DOI] [PubMed] [Google Scholar]
- 28.Fonarow G.C., Wright R.S., Spencer F.A. Effect of statin use within the first 24 hours of admission for acute myocardial infarction on early morbidity and mortality. Am J Cardiol. 2005;96:611–616. doi: 10.1016/j.amjcard.2005.04.029. [DOI] [PubMed] [Google Scholar]
- 29.Ludman A., Venugopal V., Yellon D.M., Hausenloy D.J. Statins and cardioprotection—more than just lipid lowering? Pharmacology & Therapeutics. 2009;122:30–43. doi: 10.1016/j.pharmthera.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 30.Lighter J., Phillips M., Hochman S. Obesity in patients younger than 60 years is a risk factor for COVID-19 hospital admission. Clin Infect Dis. 2020 Apr 9 doi: 10.1093/cid/ciaa415. [E-pub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.