Abstract
A 50-year-old male presented with atrial flutter 25 days after heart and kidney transplantation. Rejection was excluded, but he developed severe COVID-19 infection with cardiac allograft dysfunction. Despite continued corticosteroid and tacrolimus therapy, he remained aviremic. Respiratory and myocardial functions recovered after a week of mechanical ventilation. The cardiomyopathy was stress induced. (Level of Difficulty: Advanced.)
Key Words: cardiomyopathy, COVID-19, heart transplantation, SARS-CoV-2
Abbreviations and Acronyms: AMR, antibody-mediated rejection; COVID-19, coronavirus 2019; CRP, C-reactive protein; LVAD, left ventricular assist device; SARS-CoV-2, severe acute respiratory syndrome-coronavirus-2
Graphical abstract
A 50-year-old male presented with atrial flutter 25 days after heart and kidney transplantation. Rejection was excluded, but he developed severe COVID-19…
Presentation
A 50-year-old African-American male presented 25 days after receiving combined heart and kidney transplants with asymptomatic atrial flutter. He converted spontaneously to sinus rhythm. Blood pressure was 128/75 mm Hg; heart rate was 95 beats/min; temperature was 36.8°C. Cardiopulmonary examination was normal. Endomyocardial biopsy was negative for rejection. Hemodynamics were normal, and graft function was hyperdynamic.
Learning Objectives
-
•
To be able to arrive at the differential diagnosis of acute cardiac dysfunction in the setting of severe COVID-19 disease early after heart transplantation.
-
•
To understand the need for individualized management, balancing risks of infection and rejection in heart transplantation recipients with severe infections early after transplantation.
The patient developed cough and rhinorrhea 48 h later. Nasopharyngeal swab sample was positive for severe acute respiratory syndrome-coronavirus-2 (SARS-CoV-2) infection. He had no dyspnea. Chest radiograph findings and pulse oximetry on room air were normal. His prednisone dose had just been reduced to 30 mg after the biopsy. Tacrolimus was continued, and mycophenolate dose was reduced by 50%. Hydroxychloroquine was started. After 3 days of being monitored with no clinical change, the patient was discharged to isolation at home with pulse oximetry and plans for daily telemedicine assessments.
Three days later the patient presented to the emergency department with rapidly progressive respiratory distress and hypoxia.
Medical History
The patient had a history of end-stage nonischemic cardiomyopathy, diabetes, and chronic kidney disease. Previously he underwent implantation of a left ventricular assist device (LVAD) as a bridge to cardiac transplantation, which was complicated by late-onset post-LVAD right ventricular dysfunction with inotrope dependence and severe cardiorenal syndrome 2 years later. Due to recurrent hospitalizations for intractable hypervolemia deemed nondischargeable, he was approved by the United Network of Organ Sharing board as a status-2 criterion exemption for heart transplantation. He underwent heart transplantation on February 28, 2020, followed by renal transplantation the following day, both without complications. Basiliximab was used to induce immunosuppression, followed by maintenance with prednisone, tacrolimus, and mycophenolate mofetil.
Differential diagnosis
Diagnoses included SARS-CoV-2 infection disease, heart transplant rejection, viral myocarditis, and stress cardiomyopathy.
Investigation
Chest radiography showed extensive bilateral airspace opacities. Laboratory values were significant for elevated ferritin concentration at 648 ng/ml, C-reactive protein concentration of 74.9 mg/l, and D-dimer of 4.81 μg/ml, raising concern for cytokine release syndrome. Electrocardiography showed sinus rhythm with no ST-segment changes. High-sensitivity troponin concentration was mildly elevated (34 pg/ml), with a flat trend. Transthoracic echocardiography demonstrated new biventricular systolic dysfunction with left ventricle ejection fraction of 40% and moderately depressed right ventricular function. A test for serum SARS-CoV-2 PCR infection performed through a research pathway was negative.
Management
The patient was admitted to the intensive care where he required intubation and mechanical ventilation. His lowest partial pressure of arterial oxygen-to-percentage of inspired oxygen (Pao2/Fio2) ratio was 116. Lung protective ventilation along with deep sedation, paralytics, and inhaled epoprostenol were started for severe acute respiratory distress syndrome. He was excluded from randomized treatment trials due to immunosuppression but met institutional criteria for compassionate off-label use of tocilizumab. One 400-mg intravenous (IV) dose was administered after risk-benefit analysis and consent of the patient’s legal representative. He received azithromycin and broad-spectrum antibiotics for possible hospital-acquired pneumonia, and hydroxychloroquine was continued with daily QTc monitoring. Prednisone was switched to stress-dose IV hydrocortisone. Tacrolimus was switched to continuous infusion with a target steady-state level of 10 to 12. Mycophenolate was discontinued.
Lung protective ventilation was used, and he required 12 h of paralysis. His Pao2/Fio2 ratio remained above 150 without proning. Diuretics were given to maintain daily negative fluid balance, monitoring for hypotension or worsening renal function. He developed a mild acute kidney injury that resolved with no change in therapy. Mean arterial pressure and other end-organ functions remained normal throughout his course without vasopressors or inotropes. Respiratory failure improved gradually, and he was extubated on day 7 and taken off oxygen therapy by day 12. A repeat transthoracic echocardiogram performed on day 8 showed normalization of biventricular function. He was discharged on hospital day 14.
Discussion
Since the emergence of SARS-CoV-2 from coronavirus-2019 (COVID-19) infection and declaration of the disease as a global pandemic, immunocompromised and solid organ transplant recipients have been considered a vulnerable population with projected worse outcomes (1). Data for presentation and management and prognosis of COVID-19 disease in solid organ transplant recipients are scarce (2, 3, 4). At the time the present patient underwent transplantation, no COVID-19 cases had been reported in the authors’ state. In this case, the risk-benefit ratio of heart transplantation during the evolving pandemic was believed to favor proceeding with transplantation due to the high mortality associated with his LVAD complications. To the author’s knowledge, this is the first reported case of severe COVID-19 infection diagnosed within 30 days of heart transplantation, complicated by allograft dysfunction in the setting of a recent negative biopsy. The concomitant graft dysfunction presented a diagnostic and therapeutic dilemma. The differential included rejection, the most common cause in the first year after transplantation, as well as nonrejection mechanisms, specifically viral myocarditis or stress cardiomyopathy, both of which have been described in COVID-19 disease (5,6). The approach to management would vary greatly depending on the leading consideration, as treatment for rejection may be contradictory to that of a severe viral infection.
A repeat biopsy was deferred in the absence of overt hemodynamic instability. With no episodes of graft dysfunction or rejection prior to this hospitalization, stress cardiomyopathy or myocarditis was favored over rejection as the cause. According to recent International Society for Heart and Lung Transplantation recommendations to centers treating cases of severe COVID-19 disease in cardiothoracic transplantation recipients, mycophenolate was discontinued, and a single dose of interleukin-6 inhibitor, tocilizumab, was administered. Steroids were changed to stress dosing based on consensus opinion of the resident multidisciplinary team formed by pulmonary, transplant cardiology, and infectious disease specialists. Preventing refractory hypoxemia would be a major determinant of survival. Diuretics were given to treat any superimposed cardiogenic pulmonary edema complicating acute respiratory distress syndrome. Flolan was used to support oxygenation in the setting of severe respiratory failure and depressed right ventricular function.
The significance of having paroxysmal atrial flutter 2 days prior to the diagnosis of COVID-19 disease is unclear. The patient had no arrhythmia episodes after transplantation until that point and no reversible causes were identified.
A biopsy performed at the time of arrhythmia showed no rejection or myocarditis, and there were no detectable donor-specific antibodies. Serum COVID-19 polymerase chain reaction (PCR) testing carried out 2 days after the abnormal echocardiograph was negative. The authors concluded that without any specific evidence for rejection or myocarditis, the patient most likely experienced transient stress injury to the allograft from critical illness that resolved as his condition improved.
Currently, there is no sufficient evidence to support the use of interferons, gamma globulins, antivirals, steroids, hydroxychloroquine, or tocilizumab in critically ill patients (1). Zhou et al. (7) reported predictors of in-hospital mortality in a cohort of 191 patients in Wuhan, China with confirmed COVID-19 disease. The mortality rate in patients with respiratory failure was 98%, and a serum D-dimer concentration >1 μg/ml was an independent risk factor for in-hospital death. Significant improvement in the present patient’s respiratory status was temporally related to treatment with tocilizumab and stress-dose steroids during the critical illness phase. However, the present authors are unable to attribute the patient’s improvement to these specific measures as they occurred simultaneously with standard supportive care. However, despite being on high-dose triple-agent immunosuppression and presenting with major risk factors for in-hospital mortality, he survived with excellent recovery. This supports further investigation of early use of interleukin-6 inhibitors in high-risk patients with severe COVID-19 disease.
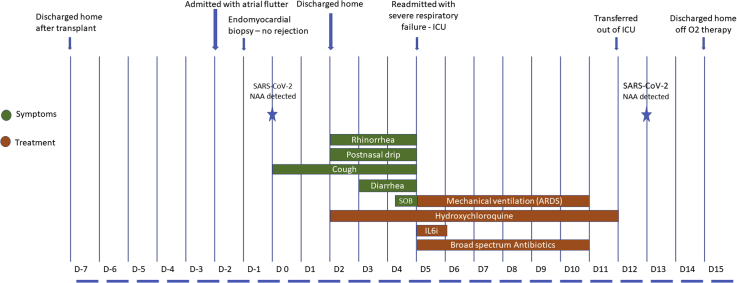
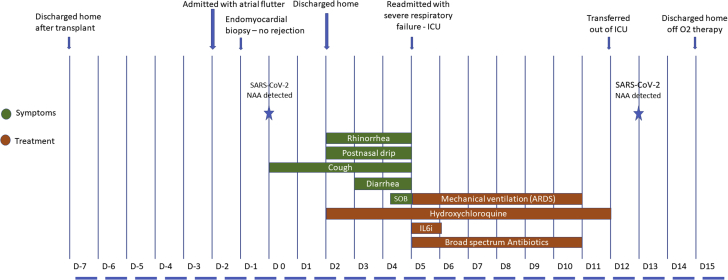
To date, there are still no reliable data to guide immunosuppression management for heart transplantation patients with COVID-19 disease. Complete withdrawal of immunosuppression during severe COVID-19 disease has been reported, but this patient’s date of transplantation was much more recent than that in the reported cases (3). The authors believed complete cessation of immunosuppression within 1 month of transplantation posed a prohibitively high risk of rejection, hence mycophenolate was stopped but tacrolimus was continued. The clinical course timeline is summarized in Figure 1.
Figure 1.
Clinical Course Timeline of Symptoms, Evaluation, and Management
ARDS = acute respiratory distress syndrome; D = day; IL-6i = interleukin-6 inhibitor; NAA = nucleic acid amplification; SOB = shortness of breath.
Follow-up
The patient reported continued gradual recovery by weekly telemedicine assessments after discharge. Mycophenolate was resumed. COVID-19 virus test of nasopharyngeal swab samples by PCR remained detectable 36 days after the initial diagnosis. Repeated endomyocardial biopsy is planned once PCR results become negative.
Conclusions
This case shows that severe COVID-19 disease is a survivable illness despite intense immunosuppression regimens in patients who are treated early after heart transplantation. The investigation identified stress cardiomyopathy among the differential for allograft dysfunction, complicating COVID-19 disease treatment early after heart transplantation, highlighting complete reversibility in the short term if the patient recovers.
Acknowledgments
The authors acknowledge Mohammad Elzeneini, MD, for assistance in creating the paper's figure, and Adam Hafeez, MD, for care provided to this patient.
Footnotes
Dr. Aranda is a consultant for Zoll LifeVest. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the JACC: Case Reportsauthor instructions page.
References
- 1.Poston J.T., Patel B.K., Davis A.M. Management of critically ill adults with COVID-19. JAMA. 2020 Mar 26 doi: 10.1001/jama.2020.4914. [E-pub ahead of print] [DOI] [PubMed] [Google Scholar]
- 2.Fried J.A., Ramasubbu K., Bhatt R. The variety of cardiovascular presentations of COVID-19. Circulation. 2020;141:1930–1936. doi: 10.1161/CIRCULATIONAHA.120.047164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li F., Cai J., Dong N. First cases of COVID-19 in heart transplantation from China. J Heart Lung Transplant. 2020;39:496–497. doi: 10.1016/j.healun.2020.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ren Z.-L., Hu R., Wang Z.-W. Epidemiological and clinical characteristics of heart transplant recipients during the 2019 coronavirus outbreak in Wuhan, China: a descriptive survey report. J Heart Lung Transplant. 2020;39:412–417. doi: 10.1016/j.healun.2020.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Meyer P., Degrauwe S., Delden C.V., Ghadri J.-R., Templin C. Typical Takotsubo syndrome triggered by SARS-CoV-2 infection. Eur Heart J. 2020;41:1860. doi: 10.1093/eurheartj/ehaa306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tavazzi G., Pellegrini C., Maurelli M. Myocardial localization of coronavirus in COVID-19 cardiogenic shock. Eur J Heart Fail. 2020;22:911–915. doi: 10.1002/ejhf.1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhou F., Yu T., Du R. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]