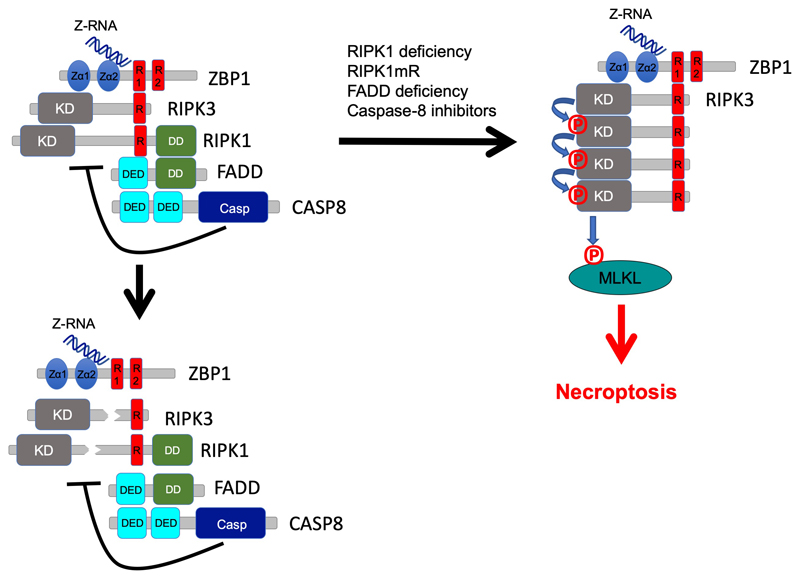
Extended Data Figure 10. Schematic model depicting the regulation of ZBP1-mediated activation of RIPK3-MLKL-dependent necroptosis by RIPK1 and caspase-8.
Sensing of endogenous cellular Z-RNA by its Zα domains activates ZBP1 inducing its interaction with RIPK3, but cell death is inhibited due to negative regulation by RIPK1 and caspase-8. RIPK1 inhibits ZBP1-induced activation of RIPK3 by FADD-mediated recruitment of caspase-8, which cleaves components of the complex such as RIPK1 and RIPK3. In cells lacking RIPK1 or expressing RIPK1 with mutated RHIM, in FADD-deficient cells as well as in cells treated with caspase inhibitors, Zα-dependent sensing of endogenous Z-RNA activates ZBP1 that strongly engages RIPK3 triggering MLKL-dependent necroptosis.