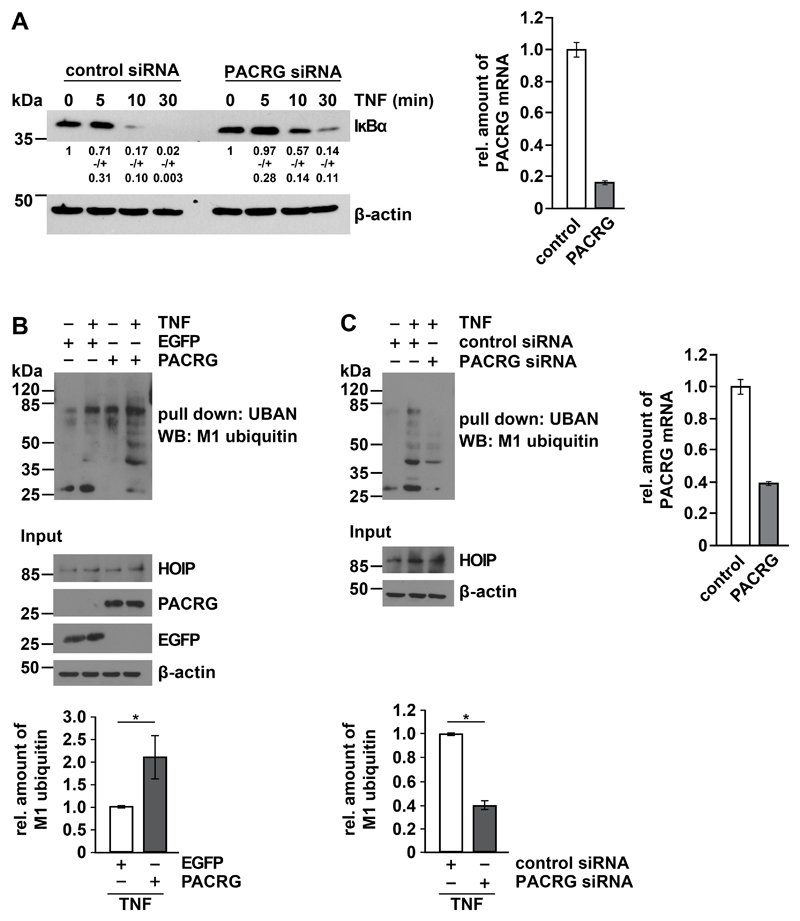
Fig. 3. PACRG increases TNF-induced linear ubiquitylation.
(A) HEK293T cells transfected with control or PACRG-specific siRNA were treated with TNF one day after transfection for the indicated times. Degradation of IκBα was determined by Western blotting for IκBα. Numbers below the IκBα-reactive bands indicate the mean value of 3 independent experiments normalized to β-actin levels +/- SEM. Graph shows PACRG knockdown efficiency as determined by real-time RT-PCR. (B) HEK293T cells were transiently transfected with plasmids encoding the indicated proteins. One day after transfection, cells were treated with TNF and lysed. Lysates generated under denaturing conditions were subjected to pull-down with the Strep-tagged UBAN domain of NEMO, and proteins affinity-purified by Strep-Tactin beads were analyzed by immunoblotting with an antibody specific for M1-linked ubiquitin. The input was immunoblotted for HOIP, PACRG, EGFP, and β-actin. Signal intensities of 3 independent experiments were quantified and normalized to the amount of linear ubiquitin chains in samples with EGFP overexpression and TNF treatment. Data represent the mean ± SEM of 3 independent experiments. For statistical analysis one-tailed Mann-Whitney U-test was performed. (C) HEK293T cells were transiently transfected with the indicated siRNAs. TNF treatment, lysis, and pull-down were performed as in (B). Signal intensities of 3 independent experiments were quantified and normalized to the amount of linear ubiquitin chains in samples treated with control siRNA and TNF. PACRG knockdown efficiency was determined by real-time RT-PCR. Data represent the mean ± SEM of 3 independent experiments. For statistical analysis one-tailed Mann-Whitney U-test was performed. *p ≤ 0.05