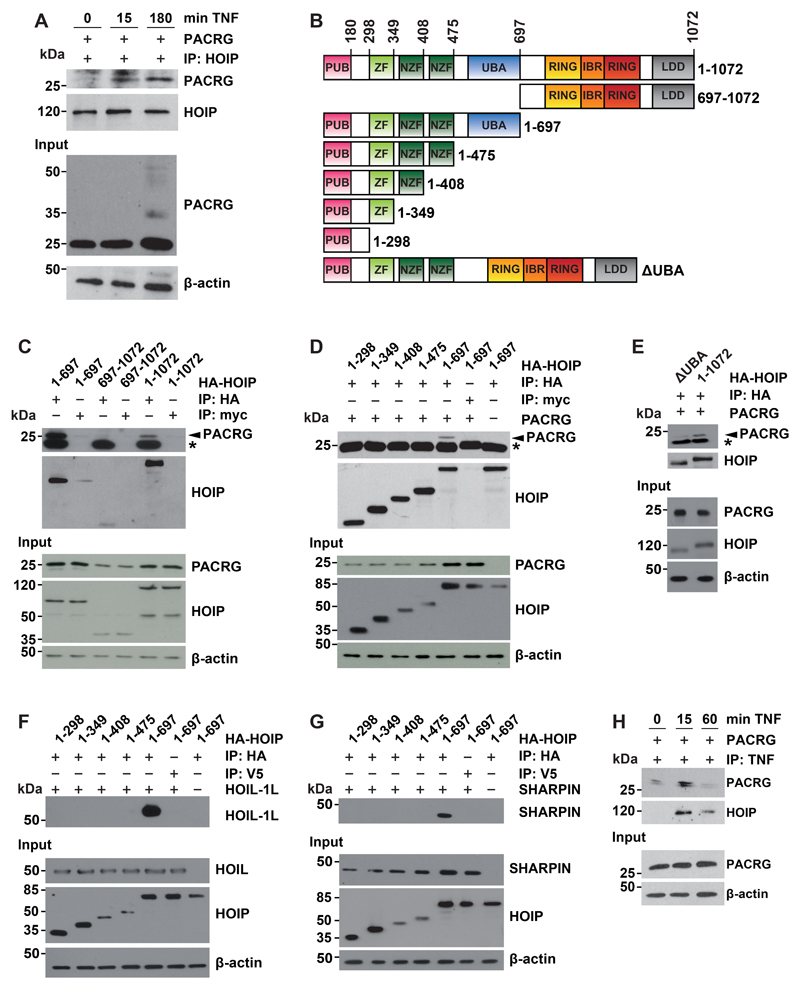
Fig. 4. PACRG binds to LUBAC.
(A) HEK293T cells were transfected with PACRG then treated with TNF for the indicated times. Cells were lysed under native conditions, and endogenous HOIP was immunoprecipitated (IP). Immunoprecipitated proteins were detected by Western Blotting for PACRG and HOIP. The input was immunoblotted for PACRG and β-actin. (B) Schematic presentation of the HOIP constructs used for immunoprecipitation. All constructs included an N-terminal HA tag. PUB, peptide N-glycosidase/ubiquitin-associated domain; ZF, zinc finger domain; NZF, nuclear protein localization 4-type zinc finger domain; UBA, ubiquitin-associated domain; RING, really interesting new gene; IBR, in-between RING domain; LDD, linear ubiquitin chain–determining domain. (C) HEK293T cells were transfected with either N-terminal (aa 1-697), C-terminal (aa 697-1072) or full-length (aa 1-1072) HA-HOIP together with PACRG. After cell lysis HOIP was immunoprecipitated and affinity purified. Co-purified PACRG was detected by Western blotting using an antibody recognizing PACRG. c-myc is a negative control. Asterisk notes a nonspecific immunoreactive band. The HOIP IP control and the HOIP input were probed with an antibody specific for HA. (D) HEK293T cells were transfected with PACRG and the indicated HOIP constructs. Co-immunoprecipitation experiments were performed as in (C). (E) HEK293T cells were transfected with either ΔUBA or full-length HA-HOIP (aa 1-1072) plus PACRG. Co-immunoprecipitation experiments were performed as in (C). (F, G) HEK293T cells were transfected with the indicated HOIP constructs and either HOIL-1L (F) or SHARPIN (G). Co-immunoprecipitation experiments were performed as in (C). HOIP input levels were detected with an antibody specific for HA. HOIL-1L, SHARPIN, and β-actin were detected with antibodies against the respective proteins. (H) HEK293T cells were transfected with PACRG then treated with FLAG-TNF for the indicated times. After cell lysis FLAG-TNF was immunoprecipitated and affinity-purified. Co-purified PACRG and HA-HOIP were detected by Western blotting for PACRG and HA, respectively. All blots are representative of at least 3 independent experiments.