Abstract
bsr‐d1, an allele encoding a transcription factor identified from the rice cultivar Digu, confers durable, broad‐spectrum resistance to infections by strains of Magnaporthe oryzae. bsr‐d1 was predicted to inhibit M. oryzae‐induced expression of Bsr‐d1 RNA and degradation of hydrogen peroxide to achieve resistance to M. oryzae. However, the global effect of biological process and molecular function on blast resistance mediated by Bsr‐d1 remains unknown. In this study, we compared transcriptomic profiling between Bsr‐d1 knockout (Bsr‐d1KO) lines and the wild type, TP309. Our study revealed that bsr‐d1 mainly regulates the redox state of plant cells, but also affects amino acid and unsaturated fatty acid metabolism. We further found that BSR‐D1 indirectly regulates salicylic acid biosynthesis, metabolism, and signal transduction downstream of the activation of H2O2 signalling in the bsr‐d1‐mediated immune response. Furthermore, we identified a novel peroxidase‐encoding gene, Perox3, as a new BSR‐D1 target gene that reduces resistance to M. oryzae when overexpressed in TP309. These results provide new insights into the bsr‐d1‐mediated blast resistance.
Keywords: hydrogen peroxide (H2O2), Magnaporthe oryzae, peroxidase, resistance, rice blast, salicylic acid, transcriptome analysis
BSR‐D1 mainly regulates the redox state of plant cells by directly targeting another new gene, Perox3, but indirectly regulates salicylic acid biosynthesis and signal transduction after H2O2 signalling in the bsr‐d1‐mediated immune response.
1. INTRODUCTION
Plants employ two main layers of immune responses, pathogen‐associated molecular pattern (PAMP)‐triggered immunity (PTI) and effector‐triggered immunity (ETI), to defend against pathogens. PTI is mediated by pattern recognition receptors (PRRs) localized on cell membranes whereas ETI is triggered by nucleotide‐binding oligomerization domain‐like receptors (NLRs) localized in cytoplasm (Jones and Dangl, 2006). These immune responses trigger many molecular events, including activation of mitogen‐activated protein kinases (MAPKs), induction of defence gene expression, production of defence signal molecules, reactive oxygen species (ROS) (Kaku et al., 2006), and phytohormones (De Vleesschauwer et al., 2013), and production of antimicrobial chemicals like phytoalexins and phytoanticipins (Dixon, 2001). To date, many important defence‐related genes have been extensively studied in plants, such as Arabidopsis RPW8.2 (Xiao et al., 2001), rice Xa21 (Song et al., 1995) and bsr‐d1 (Li et al., 2017), wheat Yr36 (Fu et al., 2009), and barley mlo (Büschges et al., 1997). MicroRNAs also play important roles in plant defence, such as Arabidopsis miR393 (Navarro et al., 2006), and tomato miR482 and miR2118 (Shivaprasad et al., 2012). In response to the rice blast fungus, members of 10 miRNA families positively or negatively regulate rice defence against Magnaporthe oryzae, such as miR160, miR164, miR166, miR167, miR169, miR319, miR396, miR398, miR444, and miR7695 (Li et al., 2019b). Analyses of their signalling mechanisms and pathways have provided great insights into plant defence responses.
Rice blast, caused by M. oryzae, is a devastating disease and has been under extensive study. As a result, the rice–M. oryzae pathosystem has become a successful premier model for studying the molecular basis of plant–fungal interactions (Li et al., 2019a). Currently, some atypical resistance (R) genes, such as pi21 (Fukuoka et al., 2009) and Ptr (Zhao et al., 2018), have attracted much attention. Pi21 encodes a proline‐rich protein containing a metal‐binding domain and a loss‐of‐function allele (pi21) confers nonrace‐specific, durable resistance (Fukuoka et al., 2009). Ptr, encoding a protein with four Armadillo repeats, is required for broad‐spectrum blast resistance mediated by the R gene Pi‐ta and by the associated R gene Pi‐ta2 (Zhao et al., 2018). These findings reveal the sophistication of plant genes involved in plant innate immunity to rice blast fungus. Transcriptomic profiling analysis is a useful tool that facilitates our understanding of the change in global gene expression and has greatly assisted the study of plant immune responses. For example, it has been applied to the study of the rice–M. oryzae interaction, including using rice varieties Digu and Gigante Vercelli, which carry broad‐spectrum resistance (Bagnaresi et al., 2012; Li et al., 2016), and varieties that carry race‐specific R genes such as Pi‐k (Li et al., 2006), Pi33 (Vergne et al., 2007), Pi1, and Pi9 (Wei et al., 2013). These studies have identified several biological processes, including “response to oxidative stress”, “carbohydrate metabolic process”, “response to biotic stimulus (fungus)”, and “extracellular region” (Bagnaresi et al., 2012; Wang et al., 2014; Li et al., 2016), that are involved in immune response against blast.
Hydrogen peroxide (H2O2) and salicylic acid (SA) are important signalling molecules in plant defence systems (De Vleesschauwer et al., 2013; Cerny et al., 2018). H2O2, being relatively stable, is the predominant ROS involved in mediating the response to biotic stress conditions (Mhamdi and Van Breusegem, 2018) and has received much research attention (Cerny et al., 2018). SA is one of the key phytohormones involved in biotic stress adaptation (De Vleesschauwer et al., 2013). SA and H2O2 signalling may interplay. For example, ectopic expression of the SA‐hydroxylase transgene in tobacco impairs the hypersensitive response (HR) to pathogens mediated by H2O2 (Mur et al., 1997); H2O2 production in cell organelles induces SA biosynthesis, and leads to protective mechanisms such as stomatal closure and cell death (Saxena et al., 2016). Elevated H2O2 levels induce SA accumulation, while salicylate increases H2O2 levels in plants (Chamnongpol et al., 1998). However, which of SA and H2O2 is the primary contributing factor to enhanced resistance to pathogens sometimes remains a contentious issue.
Currently, more than 70 defence‐related genes that contribute to or regulate blast resistance have been identified; their encoded proteins are dispersed in the whole cell and their signalling pathways are intertwined (Li et al., 2019a). Among these genes, Pi9 (Liu et al., 2002), Pigm (Deng et al., 2017), pi21 (Fukuoka et al., 2009), and bsr‐d1 (Li et al., 2017) are of the greatest interest because they confer broad‐spectrum blast resistance and bring little or no significant yield penalty. bsr‐d1, a natural, recessive allele from the rice variety Digu, encodes a C2H2‐type transcription factor that directly regulates the expression levels of two peroxidase genes to modulate the rice immune response to M. oryzae.
Here, we assessed the global effects of BSR‐D1 by comparing the transcriptomic profiles of Bsr‐d1 knockout (Bsr‐d1KO) and the wild‐type TP309, and found that bsr‐d1 regulates the redox state of cells, amino acid metabolism, and unsaturated fatty acid metabolic processes. Meanwhile, we found that H2O2 signalling occurs prior to SA signalling in the blast disease resistance mediated by bsr‐d1. In addition to the two previously identified peroxidase genes, we further identified a new BSR‐D1 target gene, Perox3 (LOC_Os01g73170), which confers enhanced susceptibility to M. oryzae when overexpressed in TP309.
2. RESULTS
2.1. The global effects of BSR‐D1 in the redox state of rice, and amino acid and unsaturated fatty acid metabolic processes
To assess the global effects of BSD‐D1, we first compared the gene expression profiles of Bsr‐d1KO lines, which mimic bsr‐d1 action conferring enhanced resistance, and the wild type, TP309, and identified a total of 164 differentially expressed genes (DEGs) (Figure 1a). Fifty DEGs were up‐regulated, whereas 114 DEGs were down‐regulated in Bsr‐d1KO, indicating a change in expression of a relatively limited number of genes on knockout of Bsr‐d1.
FIGURE 1.
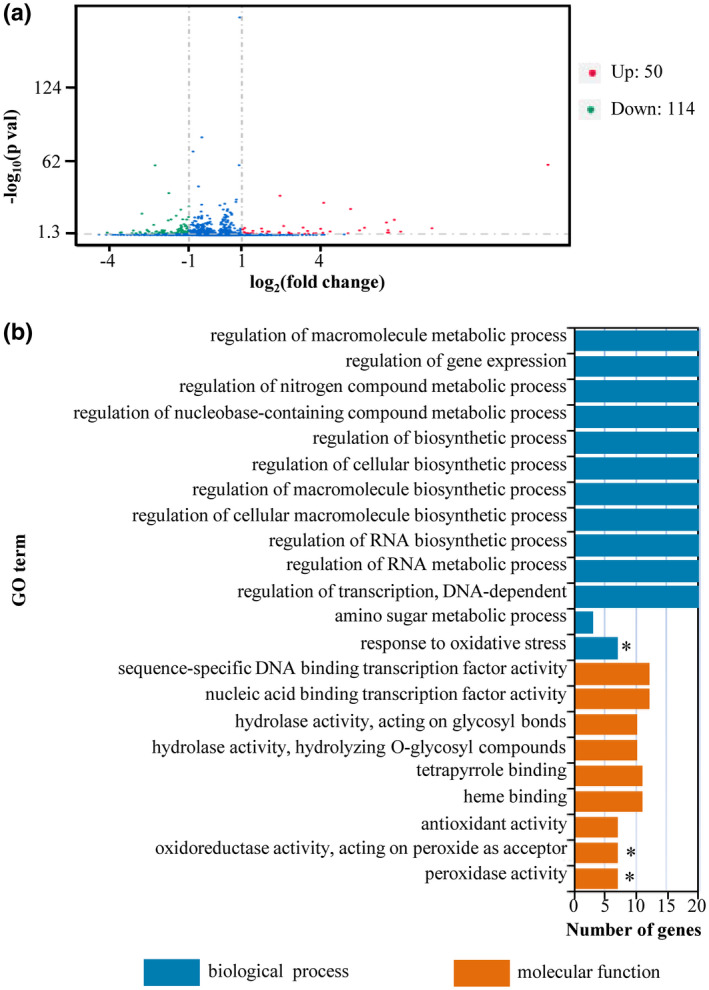
Identification of differentially expressed genes (DEGs) and GO enrichment analysis of Bsr‐d1 knockout lines (Bsr‐d1KO) and rice TP309. (a) Identification of DEGs from Bsr‐d1KO. Those genes with expression levels increased or decreased by more than 2‐fold in Bsr‐d1KO compared with TP309 were identified as DEGs. (b) GO enrichment analysis of DEGs in Bsr‐d1KO. Asterisks represent significant differences (p < .01)
To identify the molecular pathways that are specifically involved in Bsr‐d1KO lines, we performed gene ontology (GO) analysis on all DEGs. We identified 22 enriched GO terms in Bsr‐d1KO plants (Figure 1b). Among them, only three GO terms, namely peroxidase activity (GO: 0,004,601), oxidoreductase activity acting on peroxide as acceptor (GO: 0,016,684), and response to oxidative stress (GO: 0,006,979), were highly significantly enriched (p < .01). Interestingly, these three GO terms are all associated with reduction–oxidation reaction regulating the redox state of cells containing the same seven enriched DEGs (LOC_Os07g48010, LOC_Os01g73170, LOC_Os07g48020, LOC_Os07g48050, LOC_Os04g59150, LOC_Os04g59200, LOC_Os01g22249). These results suggest that Bsr‐d1 closely regulates the redox state of the cell.
To better understand the molecular pathways associated with the GO terms, we also analysed metabolic processes in Bsr‐d1KO lines. We identified a total of 20 metabolic pathways that are affected by Bsr‐d1 knockout (Figure 2). These pathways are mainly associated with amino acid (phenylalanine, cysteine, and methionine) and unsaturated fatty acid (α‐linolenic acid and linoleic acid) metabolic processes (Figure 2). These results indicate that Bsr‐d1 regulates amino acid and unsaturated fatty acid metabolism, which is associated with energy utilization and storage.
FIGURE 2.
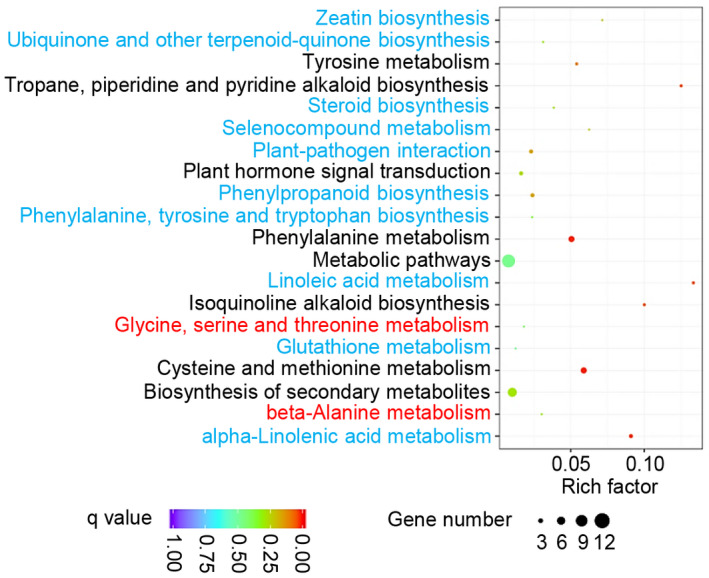
Statistics of pathway enrichment comparing Bsr‐d1 knockout (Bsr‐d1KO) plants with the wild type TP309. Red font depicts up‐regulated pathways, while blue font represents down‐regulated pathways. Additionally, black font means both up‐ and down‐regulated pathways
2.2. The role of salicylic acid in bsr‐d1 mediated blast resistance
Bsr‐d1 regulates the cellular redox state, which often cross‐talks with signalling of hormones such as SA, jasmonic acid (JA), and abscisic acid (ABA) when plants defend against pathogens (De Vleesschauwer et al., 2013; Cerny et al., 2018). Therefore, we analysed changes in hormone signalling pathways in Bsr‐d1KO. We found that three DEGs in the “plant hormone signal transduction” pathway were affected by Bsr‐d1 knockout. Two of the three DEGs are associated with SA signal transduction, whereas the third gene is associated with indoleacetic acid (IAA) signal transduction. Previous studies showed that M. oryzae inoculation activates the SA signal‐transduction cascade (Shimono et al., 2007). These data suggest that SA signalling is probably involved in the bsr‐d1‐mediated immunity to M. oryzae. Therefore, we assessed the expression levels of those genes involved in SA biosynthesis and signal transduction (Figure 3a). We found that the SA biosynthesis gene OsICS was induced, whereas OsPAL remained unchanged in Bsr‐d1KO plants; OsSSI2, whose product inhibits SA biosynthesis, remained unchanged. Meanwhile, OsSGT1, whose gene product catalyses the conversion of free SA into SA‐O‐β‐glucoside (SAG), was induced in Bsr‐d1KO plants. Three important genes in SA signal transduction, OsWRKY13, OsWRKY45, and OsNPR1, were all induced in Bsr‐d1KO plants (Figure 5a). The results indicate that Bsr‐d1 negatively regulates SA biosynthesis, metabolism, and signal transduction.
FIGURE 3.
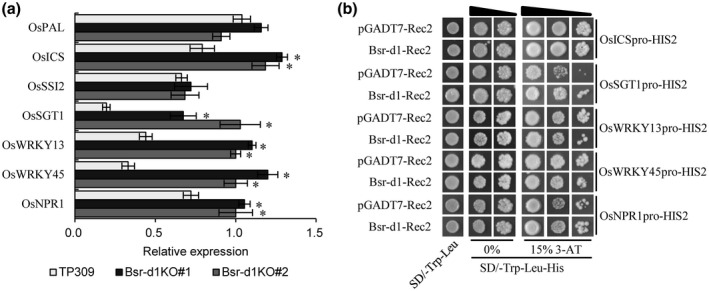
Assessment of the relationship between salicylic acid (SA) signalling and BSR‐D1. (a) Expression levels of the genes involved in SA biosynthesis, metabolism, and signal transduction in Bsr‐d1 knockout lines (Bsr‐d1KO) and the wild type TP309 were determined by quantitative reverse transcription PCR. Expression levels are normalized with the Ubq5 reference gene. RNA was prepared from leaf samples at the three‐leaf stage. Error bars represent the SD from three replicates. Asterisks represent significant differences (*p < .01). (b) Binding of BSR‐D1 to the promoters of five SA biosynthesis, metabolism, and signal transduction genes in yeast one‐hybrid assay. Each promoter was individually fused to the pHIS2 reporter and BSR‐D1 was fused to GAL4 AD. Yeast cells transformed with each of the reporter constructs and an effector construct with or without Bsr‐d1
FIGURE 5.
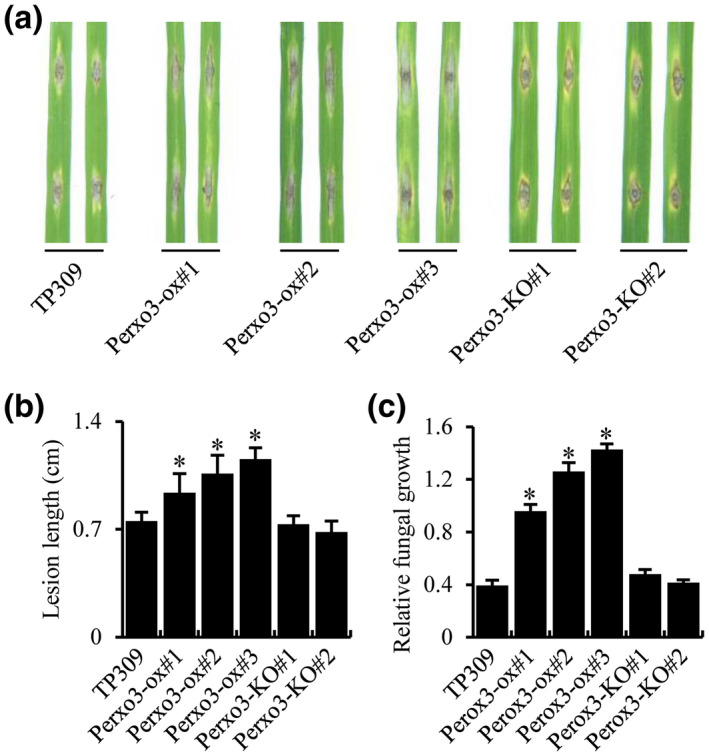
Role of Perox3 on blast resistance. (a) Punch inoculation of Perox3 overexpression (Perox3‐ox) plants. Two leaves each of Perox3‐ox #1, #2, and #3, Perox3‐KO #1 and #2, and the wild type TP309 are shown. Detached leaves of 3‐week‐old plants were punch‐inoculated. (b) Quantification of lesion length of each sample in (a). (c) Determination of blast fungal biomass. Fungal growth was determined on inoculated leaves at 6 days post‐inoculation. Fungal biomass, measured as MoPot2 by quantitative PCR, in the inoculated leaves was normalized to OsUbq DNA. The blast isolate ZB15 was used for inoculations. Error bars represent SD from three replications. Asterisks represent significant differences (*p < .01)
Both H2O2 and SA are involved in plant immune reactions (De Vleesschauwer et al., 2013; Li et al., 2017). However, there is still sometimes controversy concerning the hierarchy of H2O2 and SA in the signalling leading to disease resistance. To assess their relationship in blast disease resistance mediated by Bsr‐d1 knockout, we asked whether BSR‐D1 could bind to the promoters of the genes involved in SA biosynthesis, metabolism, or signal transduction, and activate or regulate these genes. We first determined whether BSR‐D1 could bind to the promoter of each of the OsICS, OsSGT1, OsWRKY13, OsWRKY45, and OsNPR1 genes in the yeast one‐hybrid assay. Our results show that the presence of BSR‐D1 did not lead to activation of the HIS2 reporter when each promoter was fused to the HIS2 reporter gene (Figure 3b). This suggests that BSR‐D1 in general does not directly bind to the promoters of these genes and thus indirectly regulates those genes involved in SA biosynthesis, metabolism, or signal transduction.
2.3. Identification of BSR‐D1 binding target
Our transcriptomic profiling identified seven DEGs that can potentially regulate the cell redox state. Previously, two BSR‐D1 target genes (LOC_Os05g04470 and LOC_Os10g39170) were identified that encode peroxidases (Li et al., 2017). However, we do not know whether these seven newly identified DEGs are target genes of BSR‐D1 or not. In order to assess whether they are targets of the BSR‐D1 protein, we tested binding of BSR‐D1 to the promoter of each of the above seven genes in the yeast one‐hybrid assay in which BSR‐D1 was fused to GAL4 AD and each promoter fused to the HIS2 reporter gene. Our results showed that BSR‐D1‐GAL4 AD only bound to the promoter of LOC_Os01g73170 (hereby named Perox3) because specific activation of the HIS2 reporter only occurred to Perox3 in the presence of BSR‐D1; several other candidates showed autonomous activation of the HIS2 reporter in the absence of BSR‐D1 (Figure 4a). Meanwhile, the ChIP‐seq results were also validated by carrying out real‐time PCRs to quantify the presence of these promoters. These promoters were pulled down approximately 2‐fold more frequently compared to the control, which had no antibodies added (Figure 4b). The results suggest that the BSR‐D1 protein binds to the Perox3 promoter and activates Perox3 expression. This result suggests that BSR‐D1 may indirectly regulate the other six DEGs.
FIGURE 4.
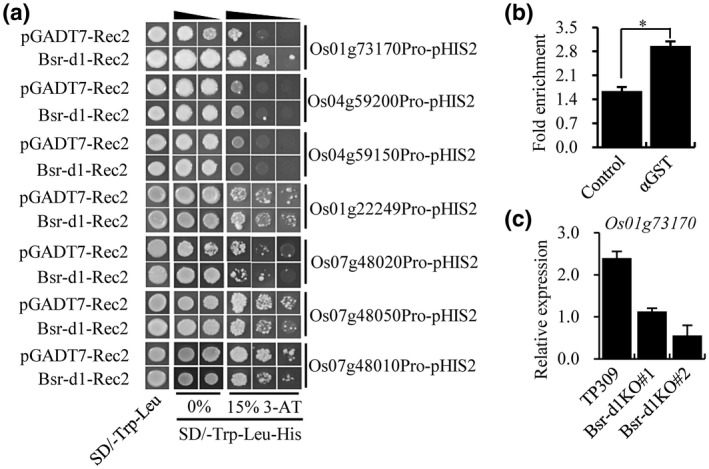
Identification of a new peroxidase gene as a direct target of BSR‐D1. (a) Binding of BSR‐D1 to the promoters of seven reduction‐oxidation reaction‐associated genes in a yeast one‐hybrid assay. Each promoter was fused to the pHIS2 reporter and BSR‐D1 was fused to GAL4 AD. Yeast cells were transformed with the reporter and effector constructs with or without Bsr‐d1. (b) In vitro pull‐down of target DNA by BSR‐D1. GST‐BSR‐D1 or GST alone were incubated with total rice DNA and subjected to quantitative PCR for the Perox3 gene. The fold enrichment was normalized against the Ub promoter. Each bar represents the mean and SD of three repeats. *p < .01. (c) RNA expression levels of the peroxidase gene (LOC_Os01g73170, named as Perox3) in Bsr‐d1 knockout (Bsr‐d1KO) plants. The expression levels are normalized to the Ubq5 reference gene. RNA was prepared from leaf samples at the three‐leaf stage. Error bars represent the SD from three replicates
To evaluate whether or not Bsr‐d1 directly regulates Perox3 expression in planta, we assessed the RNA expression level of Perox3 in the Bsr‐d1KO lines by reverse transcription quantitative PCR (RT‐qPCR). The results showed that the Perox3 RNA level was reduced 2‐ to 4‐fold compared to wild type (Figure 4c), suggesting that BSR‐D1 directly binds to the Perox3 promoter and activates Perox3 expression in rice.
2.4. Validation of the role of Perox3 in blast disease resistance
To assess the involvement of the Perox3 gene in blast disease resistance, we generated transgenic rice plants that had the Perox3 gene either overexpressed or knocked out. It is hard to accurately assay degree resistance in a resistant or hypersusceptible background. Therefore, we obtained three Perox3 overexpression (Perox3‐ox) lines in the TP309 background, which is moderately susceptible (not resistant or hypersusceptible) to M. oryzae isolate ZB15, and confirmed their elevated Perox3 RNA levels (Figure S1a). Meanwhile, we used CRISPR/Cas9 technology to knock out the endogenous Perox3 gene (Perox3‐KO) in TP309. We selected a 23‐nt sequence in the Perox3 gene as the target site for Cas9 cleavage (Figure S1b), generated multiple putative transgenic lines, and verified its knockout by sequencing. We found two lines (named Perox3‐KO#1 and #2) containing the mutation in the target site; Perox3‐KO#1 and #2 each carry a one‐base insertion in the target site (Figure S1b), truncating the Perox3 open reading frame.
Perox3‐ox and Perox3‐KO lines were challenged with the ZB15 blast isolate by punch‐inoculation using detached leaves. These three Perox3‐ox lines developed blast lesions approximately 30%–90% longer than TP309 (Figure 5a,b), indicating higher susceptibility. To confirm these lesion length results, we measured the amount of M. oryzae present in each inoculated leaf by quantifying fungal DNA. The fungal DNA quantification results also showed that the Perox3‐ox lines harboured more fungus than TP309 (Figure 5c). This elevated blast susceptibility phenotype cosegregated with the presence of the transgene when assessed in a segregating progeny population (Figure S2). However, the two Perox3‐KO lines developed similar lesions as TP309 (Figure 5a,b); fungal DNA quantification also showed that the Perox3‐KO lines harboured similar amounts of M. oryzae as TP309 (Figure 5c). These results may suggest that there are redundant genes of Perox3, such as the two previously identified peroxidases, and disruption of Perox3 alone does not lead to an observable phenotypic change. Thus, the results of the overexpression experiment suggest that the Perox3 gene functions as a regulator to blast resistance with redundant genes.
3. DISCUSSION
We have previously reported the identification of the bsr‐d1 allele that confers broad‐spectrum blast resistance and the discovery of its underlying mechanism (Li et al ., 2017). Here, we have further conducted a transcriptomic analysis to assess the global effect of knocking out the Bsr‐d1 gene, which mimics the effect of bsr‐d1, and found that redox regulatory genes, including a novel peroxidase gene Perox3, are the primary targets of BSR‐D1. We empirically confirmed the binding of BSR‐D1 to the Perox3 promoter. We also determined that genes involved in SA biosynthesis, metabolism, and signalling are indirectly regulated by BSR‐D1, downstream of the regulation of H2O2 levels and H2O2 signal transduction. Thus, our findings significantly advance our understanding of the bsr‐d1‐mediated broad‐spectrum resistance to M. oryzae and have several important implications.
3.1. Degradation of H2O2 is the primary target for BSR‐D1
H2O2 is a relatively stable nonfree‐radical ROS that is involved in programmed cell death (PCD) of infected and surrounding cells under pathogen attack (Birch et al., 2018). We previously identified the Bsr‐d1 gene that directly up‐regulates peroxidase gene expression to suppress the accumulation of H2O2, impairing blast disease resistance (Li et al., 2017). Our results of transcriptomic profiling presented here further support our previous model because all three significant GO terms enriched in Bsr‐d1KO lines are associated with redox state regulation. Among the three GO terms, response to oxidative stress (GO: 0,006,979) is often specifically enriched in resistant rice varieties (Bagnaresi et al., 2012; Wang et al., 2014; Li et al., 2016), which is consistent with our model.
H2O2 is a major redox metabolite and at high concentrations induces oxidative damage to biomolecules (Cerny et al., 2018). To avoid oxidative damage to cellular structures, plants mainly use enzymatic antioxidants, such as superoxide dismutases (SODs), catalases (CATs), and peroxidases (POXs), to scavenge H2O2 (Xie et al., 2019). Our previous results showed that two peroxidase genes induced by BSR‐D1 are employed by M. oryzae to suppress blast disease resistance (Li et al., 2017). In this study, we identified a third peroxidase gene, Perox3, which also negatively regulates blast disease resistance (Figure 5). The multiple peroxidase genes involved in suppressing H2O2 accumulation may be a host cellular mechanism to safeguard the cell from oxidative damage. However, this mechanism was hijacked by M. oryzae through activation of the Bsr‐d1 gene and used to counter the ROS burst induced during the rice immune response on M. oryzae infection (Li et al ., 2017). To counter this M. oryzae strategy, the rice host Digu has developed a bsr‐d1 allele that can no longer be activated by M. oryzae blocking the activation of bsr‐d1 and its target peroxidases, leading to the accumulation of H2O2 needed for resistance to M. oryzae (Li et al ., 2017).
3.2. The role of unsaturated fatty acids in the interface between rice and M. oryzae
Multiple molecular events occur in the rice–M. oryzae interaction (Bagnaresi et al., 2012; Wei et al., 2013; Wang et al., 2014; Li et al., 2016). Here, we identified three highly significantly enriched GO terms associated with reduction–oxidation reactions regulating the redox state of cells in Bsr‐d1KO, namely peroxidase activity (GO: 0,004,601), oxidoreductase activity acting on peroxide as acceptor (GO: 0,016,684), and response to oxidative stress (GO: 0,006,979). In particular, response to oxidative stress (GO: 0,006,979) is specifically involved in blast resistance, as reported previously (Bagnaresi et al., 2012; Wang et al., 2014; Li et al., 2016), which further supports the importance of ROS in plant immunity (Mittler et al., 2004). Our results show that amino acid (phenylalanine, cysteine, and methionine) metabolic processes regulated by Bsr‐d1 might provide needed carbon and nitrogen sources for rice or M. oryzae are similar to the previously reported transcriptomic profiling results using IRBL18 (Wei et al., 2013). Additionally, Bsr‐d1 affects the unsaturated fatty acid (α‐linolenic acid and linoleic acid) metabolic processes. Previous studies have reported that there is a relationship between ROS and unsaturated fatty acids. For example, ROS, such as 1O2, directly oxidize unsaturated fatty acids (Wagner et al ., 2004). A single •OH can result in peroxidation of many polyunsaturated fatty acids and peroxidation of unsaturated fatty acids may produce malondialdehyde (MDA), which is responsible for cell membrane damage (Sharma et al., 2012). This report indicates that unsaturated fatty acids are involved in the response of defending against blast fungus. Thus, our transcriptomic results are consistent with previous reports supporting the notion that unsaturated fatty acids may be another battlefront in the interaction between rice and M. oryzae.
3.3. Signalling pathways in the bsr‐d1‐mediated defence response
Bsr‐d1 regulates some of the genes associated with SA biosynthesis, metabolism, and signal transduction (Figure 3a). However, a previous report suggested that the levels of endogenous SA do not change significantly on pathogen attack in rice (Silverman et al., 1995), though exogenously applied SA can induce resistance to M. oryzae (Iwai et al., 2007). This indicates that bsr‐d1 may regulate the SA signal transduction to achieve blast disease resistance.
H2O2 and SA are important signalling molecules in the plant immune response (De Vleesschauwer et al., 2013; Cerny et al., 2018). The two signal moleculaes can interplay (Saxena et al., 2016). For example, SA can increase H2O2 levels in plant tissues (Rao et al., 1997), while SA accumulation can also be induced by elevated H2O2 levels (Chamnongpol et al., 1998; Mhamdi et al., 2010). Arabidopsis GLUTATHIONE REDUCTASE1 plays an important role in increasing intracellular H2O2 production and SA accumulation in the Col‐0 background under long‐day conditions (Mhamdi et al., 2010). Similarly, Bsr‐d1 appears to regulate both the genes that modulate H2O2 concentration and those that are involved in SA biosynthesis, metabolism, and signal transduction (Figures 3a and 4). H2O2 can be placed upstream or downstream of SA in their signalling cascades when plants respond to different environment stresses (Chen and Kessig, 1991; Cerny et al., 2018). In Bsr‐d1‐mediated signalling, BSR‐D1 can directly bind to the promoter of peroxidase genes, but not to the promoters of the genes associated with SA biosynthesis, metabolism, and signal transduction, clearly suggesting that H2O2 signalling occurs prior to SA signalling in the blast disease resistance mediated by bsr‐d1.
4. EXPERIMENTAL PROCEDURES
4.1. Plant materials and blast infection procedures
Rice TP309 and transgenic lines including Bsr‐d1KO, Perox3‐ox, and Perox3‐KO were grown in two tubs in a growth chamber at 28 °C in a 12‐hr light/12‐hr dark photoperiod with 75% humidity. Three‐week‐old rice plants were used for inoculation with M. oryzae isolate ZB15. M. oryzae spores were grown on complete agar medium for 2 weeks before producing spores. Spores were collected via flooding of the fungal agar cultures with sterile water, and the spore concentration in the suspension was adjusted to 5 × 105 conidia/ml before punch inoculation. Punch inoculation of detached rice leaves is modified based on Jia et al. (2003) with the following modification. First, 4 μl of spore suspension was placed at each of two spots on each leaf using a micropipette. Inoculated detached leaves were placed in 0.1% 6‐benzylaminopurine (6‐BA) in sterile water to keep moist. The lesion lengths of disease reactions were measured using a ruler 5 days post‐inoculation. The relative fungal DNA amount was calculated using the threshold cycle value (C t) of M. oryzae Pot2 DNA against the C t of rice genomic ubiquitin DNA (Park et al., 2012).
4.2. Transcriptome analysis
We used leaf samples at the three‐leaf stage for RNA‐Seq in this transcriptomic study. RNA quantification and qualification, library preparation for strand transcriptome sequencing, clustering and sequencing, and part data analysis were performed at Novogene Bioinformatics Technology Co., Ltd (Tianjin, China) following the manufacturer's instructions. Differential expression analyses of two rice varieties were performed using the DESeq R package v. 1.18.0. DESeq provided statistical routines for determining differential expression in digital gene expression data using a model based on the negative binomial distribution. The resulting p values were adjusted using the Benjamini and Hochberg approach for controlling the false discovery rate. Genes with an adjusted p value <.05 found by DESeq were assigned as differentially expressed. GO enrichment analysis of DEGs was implemented by the GOseq R package, in which gene length bias was corrected. GO terms with corrected p value <.05 were considered significantly enriched DEGs. The KOBAS software was used to test the statistical enrichment of DEGs in KEGG pathways.
4.3. RNA isolation and RT‐qPCR
Total RNA was extracted using TRIzol reagent (Invitrogen Life Technologies) following the manufacturer's protocols. cDNA was synthesized using an RNA reverse transcription kit (Invitrogen Life Technologies). RT‐qPCR was conducted using a Bio‐Rad CFX96 Real‐Time System coupled to a C1000 thermal cycler (Bio‐Rad). The reference gene Ubiquitin 5 (Ubq5) was used for the normalization of all RT‐qPCR data (Li et al., 2017). The sequences of the primers are listed in Table S1. The 2−∆∆ C t method was used to calculate the relative expression levels with three technical repeats (Livak and Schmittgen, 2001).
4.4. One‐hybrid assays in yeast
The full‐length cDNA sequence of transcription factor gene Bsr‐d1 was amplified and fused in frame with the GAL4 activation domain in vector pGADT7‐Rec2 (Clontech) forming construct pGADT7‐ Bsr‐d1. Then, the fusion construct was cotransformed with the reporter construct (pHIS2‐fused to each promoter of LOC_Os04g59150, LOC_Os04g59200, LOC_Os01g73170, LOC_Os07g48010, LOC_Os01g22249, LOC_Os07g48050, LOC_Os07g48020, OsICS, OsSGT1, OsWRKY13, OsWRKY45, and OsNPR1) into Y187 yeast cells (Clontech). The sequences of the primers are listed in Table S1. The locations of each promoter for Y1H are shown in Table S2. The empty vector pGADT7‐Rec2 and the pHIS2‐ promoter were cotransformed as the negative control for mating experiments. DNA–protein interactions were determined by the growth of the transformants on the nutrient‐deficient medium with 0 and 15 mM 3‐amino‐1,2,4‐triazole (3‐AT), following the manufacturer's instructions (Clontech).
4.5. Semi‐in vivo chromatin immunoprecipitation (ChIP) and ChIP‐qPCR
Total DNA of TP309 and purified GST‐BSR‐D1 were used for ChIP assays. The protocol has been published before (Li et al., 2017). Three‐week‐old seedlings were used for total DNA extraction. The total DNA was sheared into 100–500 bp fragments using an ultrasonic crusher. The glutathione‐S‐transferase (GST) fusion protein was affinity‐purified on glutathione‐agarose beads (BD Biosciences). DNA fragments enriched by GST‐BSR‐D1 were obtained using the procedure of semi‐in vivo ChIP. The prepared DNA in ChIP was applied for qPCR using respective primer pairs (the amplified fragment with predicted conserved motif from −164 bp neighbouring start codon) (Table S1). The expression levels were normalized to the input sample for enrichment detection. The fold enrichment was calculated against the Ub promoter. No addition of antibodies (NoAbs) served as a negative control.
4.6. Plasmid construction and plant transformation
The full‐length cDNA of Perox3 was cloned into pCAMBIA2300 to generate the overexpression construct, pCAMBIA2300‐Perox3. The pCAMBIA2300‐Perox3 construct was introduced into TP309 through Agrobacterium‐mediated transformation as described previously (Li et al., 2017). The regenerated transgenic plants carrying Perox3‐ox were selected with G418. PCR‐based genotyping was performed to verify the presence of the transgene as previously described (Li et al., 2016). Overexpression of Perox3 in the transgenic lines was confirmed by RT‐qPCR.
For CRISPR (clustered regularly interspaced short palindromic repeats)/Cas9 construction, the 23 bp targeting sequence (including PAM) of Perox3 was confirmed using a BLAST search against the rice genome (https://blast.ncbi.nlm.nih.gov/Blast.cgi) (Hsu et al., 2013). The designed targeting sequence was synthesized and annealed to form the oligo adaptors. Vector pBGK032 was digested by BsaI and purified using a DNA purification kit (Tiangen). A ligation reaction (10 µl) containing 10 ng of the digested pBGK032 vector and 0.05 mM oligo adaptor was carried out and directly transformed to Escherichia coli competent cells to produce CRISPR/Cas9 plasmid. The CRISPR/Cas9 plasmids were introduced into Agrobacterium tumefaciens EHA105. Transformation of rice was performed as described above. Genomic DNA was extracted from these transformants and primer pairs flanking the designed target site were used for PCR amplification (Table S1). The PCR products (300–500 bp) were sequenced.
Supporting information
FIGURE S1 Confirmation of overexpression and knockout in Perox3 transgenic lines. (a) Determination of Perox3 RNA expression levels in the Perox3 overexpression plants. Leaf samples collected from Perox3 overexpression lines Perox3‐ox#1, Perox3‐ox#2, Perox3‐ox#3, and wild‐type TP309 at the seedling stage were subjected to RNA extraction followed by quantitative reverse transcription PCR (RT‐qPCR) analysis. RT‐qPCR results are normalized with the Ubq5 reference gene. Error bars represent SD from three replicates. Asterisks represent significant differences (*p < .01). (b) Confirmation of Perox3 knockout. The target site designed for knocking out the Perox3 gene by the CRISPR/Cas9 system. Verification of the knockout lines by PCR‐based sequencing. Two representative transgenic lines (abbreviated as Perox3‐KO#1 and Perox3‐KO#2) are generated from the TP309 genetic background
FIGURE S2 Cosegregation of enhanced susceptibility to Magnaporthe oryzae with the Perox3‐ox#1 transgene. (a) Photographs of representative leaves post punch inoculation. a1 to a10, and the TP309 control are shown. PCR‐based genotyping with a primer pair specific for the NptII gene was performed to determine whether the plants contained (+) or lacked (–) the Perox3‐ox construct. Blast isolate ZB15 was used for inoculations (conidial concentration of 5 × 105/ml). (b) Quantitation of lesion lengths from the segregants in (a)
TABLE S1 Primers used in this study
TABLE S2 The amplified part of each promoter of these genes used for one‐hybrid assay
ACKNOWLEDGEMENTS
This work was supported by the National Natural Science Foundation of China (NSFC) (31772152, 31972254), the Fok Ying Tung Education Foundation (171021), the Open Research Fund of the State Key Laboratory of Hybrid Rice (Hunan Hybrid Rice Research Center) (2017KF01), the Tianfu Ten‐thousand Talents Programme (Tianfu Science and Technology Elite Project), the Outstanding Young Scientific and Technological Talents Project in Sichuan Province (2019JDJQ0045), and the Innovative Training Programme of Sichuan Agricultural University (201910626042) to W.L.; the NSFC (31825022, 31772153 and 31571994) to X.C.; the NSFC (31871920) to M.H.; the NSFC (31601290) to J.Y.; the Applied Basic Research Programmes of the Science and Technology Department from Sichuan Province (2019YJ0432) and NSFC (31701779) to X.Z.; the National Science Foundation (1237975), the National Institutes of Health (GM59962), and USDA National Institute of Food and Agriculture (2017‐67013‐26590) to M.C.
Zhu Z, Yin J, Chern M, et al. New insights into bsr‐d1‐mediated broad‐spectrum resistance to rice blast. Molecular Plant Pathology. 2020;21:951–960. 10.1111/mpp.12941
Ziwei Zhu, Junjie Yin, Mawsheng Chern, and Xiaobo Zhu contributed equally to this work.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- Bagnaresi, P. , Biselli, C. , Orru, L. , Urso, S. , Crispino, L. , Abbruscato, P. et al (2012) Comparative transcriptome profiling of the early response to Magnaporthe oryzae in durable resistant vs susceptible rice (Oryza sativa L.) genotypes. PLoS ONE, 7, e51609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birch, P.R.J. , Avrova, A.O. , Dellagi, A. , Lacomme, C. , Cruz, S.S. and Lyon, G.D. (2018) Programmed cell death in plants in response to pathogen attack. Annual Plant Reviews, 4, 184–208. [Google Scholar]
- Büschges, R. , Hollricher, K. , Panstruga, R. , Simons, G. , Wolter, M. , Frijters, A. et al (1997) The barley Mlo gene: a novel control element of plant pathogen resistance. Cell, 5, 695–705. [DOI] [PubMed] [Google Scholar]
- Cerny, M. , Habanova, H. , Berka, M. , Luklova, M. and Brzobohaty, B. (2018) Hydrogen peroxide: its role in plant biology and crosstalk with signalling networks. International Journal of Molecular Sciences, 19, 2812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamnongpol, S. , Willekens, H. , Moeder, W. , Langebartels, C. , Sandermann, H. , Van Montagu, M. et al (1998) Defense activation and enhanced pathogen tolerance induced by H2O2 in transgenic tobacco. Proceedings of the National Academy of Sciences of the United States of America, 10, 5818–5823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, Z. and Klessig, D.F. (1991) Identification of a soluble salicylic acid‐binding protein that may function in signal transduction in the plant disease‐resistance response. Proceedings of the National Academy of Sciences of the United States of America, 88, 8179–8183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vleesschauwer, D. , Gheysen, G. and Höfte, M. (2013) Hormone defense networking in rice: tales from a different world. Trends in Plant Science, 10, 555–565. [DOI] [PubMed] [Google Scholar]
- Deng, Y. , Zhai, K. , Xie, Z. , Yang, D. , Zhu, X. , Liu, J. et al (2017) Epigenetic regulation of antagonistic receptors confers rice blast resistance with yield balance. Science, 6328, 962–965. [DOI] [PubMed] [Google Scholar]
- Dixon, R.A. (2001) Natural products and plant disease resistance. Nature, 411, 843–847. [DOI] [PubMed] [Google Scholar]
- Fu, D. , Uauy, C. , Distelfeld, A. , Blechl, A. , Epstein, L. , Chen, X. et al (2009) A kinase‐START gene confers temperature‐dependent resistance to wheat stripe rust. Science, 5919, 1357–1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuoka, S. , Saka, N. , Koga, H. , Ono, K. , Shimizu, T. , Ebana, K. et al (2009) Loss of function of a proline‐containing protein confers durable disease resistance in rice. Science, 5943, 998–1001. [DOI] [PubMed] [Google Scholar]
- Hsu, P.D. , Scott, D.A. , Weinstein, J.A. , Ran, F.A. , Konermann, S. , Agarwala, V. et al (2013) DNA targeting specificity of RNA‐guided Cas9 nucleases. Nature Biotechnology, 31, 827–832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwai, T. , Seo, S. , Mitsuhara, I. and Ohashi, Y. (2007) Probenazole‐induced accumulation of salicylic acid confers resistance to Magnaporthe grisea in adult rice plants. Plant Cell Physiology, 48, 915–924. [DOI] [PubMed] [Google Scholar]
- Jia, Y. , Valent, B. and Lee, F.N. (2003) Determination of host responses to Magnaporthe grisea on detached rice leaves using a spot inoculation method. Plant Disease, 87, 129–133. [DOI] [PubMed] [Google Scholar]
- Jones, J.D.G. and Dangl, J.L. (2006) The plant immune system. Nature, 444, 323–329. [DOI] [PubMed] [Google Scholar]
- Kaku, H. , Nishizawa, Y. , Ishii‐Minami, N. , Akimoto‐Tomiyama, C. , Dohmae, N. , Takio, K. et al (2006) Plant cells recognize chitin fragments for defense signaling through a plasma membrane receptor. Proceedings of the National Academy of Sciences of the United States of America, 103, 11086–11091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, Q. , Chen, F. , Sun, L. , Zhang, Z. , Yang, Y. and He, Z. (2006) Expression profiling of rice genes in early defense responses to blast and bacterial blight pathogens using cDNA microarray. Physiological and Molecular Plant Pathology, 68, 51–60. [Google Scholar]
- Li, W. , Liu, Y. , Wang, J. , He, M. , Zhou, X. , Yang, C. et al (2016) The durably resistant rice cultivar Digu activates defence gene expression before the full maturation of Magnaporthe oryzae appressorium. Molecular Plant Pathology, 3, 354–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, W. , Zhu, Z. , Chern, M. , Yin, J. , Yang, C. , Ran, L. et al (2017) A natural allele of a transcription factor in rice confers broad‐spectrum blast resistance. Cell, 170, 114–126. [DOI] [PubMed] [Google Scholar]
- Li, W. , Chern, M. , Yin, J. , Wang, J. and Chen, X. (2019a) Recent advances in broad‐spectrum resistance to the rice blast disease. Current Opinion in Plant Biology, 50, 114–120. [DOI] [PubMed] [Google Scholar]
- Li, Y. , Jeyakumar, J.M.J. , Feng, Q. , Zhao, Z.X. , Fan, J. , Khaskheli, M.I. et al (2019b) The roles of rice microRNAs in rice–Magnaporthe oryzae interaction. Phytopathology Research, 1, 33. [Google Scholar]
- Liu, G. , Lu, G. , Zeng, L. and Wang, G.L. (2002) Two broad‐spectrum blast resistance genes, Pi9(t) and Pi2(t), are physically linked on rice chromosome 6. Molecular Genetics and Genomics, 4, 472–480. [DOI] [PubMed] [Google Scholar]
- Livak, K.J. and Schmittgen, T.D. (2001) Analysis of relative gene expression data using real‐time quantitative PCR and the 2−ΔΔCT method. Methods, 4, 402–408. [DOI] [PubMed] [Google Scholar]
- Mhamdi, A. and Van Breusegem, F. (2018) Reactive oxygen species in plant development. Development, 145, dev164376. [DOI] [PubMed] [Google Scholar]
- Mhamdi, A. , Hager, J. , Chaouch, S. , Queval, G. , Han, Y. , Taconnat, L. et al (2010) Arabidopsis GLUTATHIONE REDUCTASE1 plays a crucial role in leaf responses to intracellular hydrogen peroxide and in ensuring appropriate gene expression through both salicylic acid and jasmonic acid signaling pathways. Plant Physiology, 153, 1144–1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittler, R. , Vanderauwera, S. , Gollery, M. and Van Breusegem, F. (2004) Reactive oxygen gene network of plants. Trends in Plant Science, 9, 490–498. [DOI] [PubMed] [Google Scholar]
- Mur, L.A.J. , Bi, Y.M. , Darby, R.M. , Firek, S. and Draper, J. (1997) Compromising early salicylic acid accumulation delays the hypersensitive response and increases viral dispersal during lesion establishment in TMV‐infected tobacco. The Plant Journal, 5, 1113–1126. [DOI] [PubMed] [Google Scholar]
- Navarro, L. , Dunoyer, P. , Jay, F. , Arnold, B. , Dharmasiri, N. , Estelle, M. et al (2006) A plant miRNA contributes to antibacterial resistance by repressing auxin signaling. Science, 312, 436–439. [DOI] [PubMed] [Google Scholar]
- Park, C. , Chen, S. , Shirsekar, G. , Zhou, B. , Khang, C.H. , Songkumarn, P. et al (2012) The Magnaporthe oryzae effector AvrPiz‐t targets the RING E3 ubiquitin ligase APIP6 to suppress pathogen‐associated molecular pattern‐triggered immunity in rice. The Plant Cell, 24, 4748–4762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao, M.V. , Paliyath, G. , Ormrod, D.P. , Murr, D.P. and Watkins, C.B. (1997) Influence of salicylic acid on H2O2 production, oxidative stress and H2O2‐metabolizing enzymes. Plant Physiology, 115, 137–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxena, I. , Srikanth, S. and Chen, Z. (2016) Cross talk between H2O2 and interacting signal molecules under plant stress response. Frontiers in Plant Science, 7, 570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma, P. , Bhushan Jha, A. , Shanker Dubey, R. and Pessarakli, M. (2012) Reactive oxygen species, oxidative damage, and antioxidative defense mechanism in plants under stressful bonditions. Journal of Botany, 2012, 1–26. [Google Scholar]
- Shimono, M. , Sugano, S. , Nakayama, A. , Jiang, C. , Ono, K. , Toki, S. et al (2007) Rice WRKY45 plays a crucial role in benzothiadiazole‐inducible blast resistance. The Plant Cell, 6, 2064–2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shivaprasad, P.V. , Chen, H.M. , Patel, K. , Bond, D.M. , Santos, B.A. and Baulcombe, D.C. (2012) A microRNA superfamily regulates nucleotide binding site‐leucine‐rich repeats and other mRNAs. The Plant Cell, 24, 859–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman, P. , Seskar, M. , Kanter, D. , Schweizer, P. , Metraux, J.P. and Raskin, I. (1995) Salicylic acid in rice (biosynthesis, conjugation, and possible role). Plant Physiology, 2, 633–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song, W.Y. , Wang, G.L. , Chen, L.L. , Kim, H.S. , Pi, L.Y. , Holsten, T. et al (1995) A receptor kinase‐like protein encoded by the rice disease resistance gene, Xa21 . Science, 5243, 1804–1806. [DOI] [PubMed] [Google Scholar]
- Vergne, E. , Ballini, E. , Marques, S. , Sidi Mammar, B. , Droc, G. , Gaillard, S. et al (2007) Early and specific gene expression triggered by rice resistance gene Pi33 in response to infection by ACE1 avirulent blast fungus. New Phytologist, 1, 159–171. [DOI] [PubMed] [Google Scholar]
- Wagner, D. , Przybyla, D. , Op Den Camp, R. , Kim, C. , Landgraf, F. , Pyo Lee, K. et al (2004) The genetic basis of singlet oxygen‐induced stress response of Arabidopsis thaliana . Science, 306, 1183–1185. [DOI] [PubMed] [Google Scholar]
- Wang, Y. , Kwon, S.J. , Wu, J. , Choi, J. , Lee, Y.H. , Agrawal, G.K. et al (2014) Transcriptome analysis of early responsive genes in rice during Magnaporthe oryzae infection. Plant Pathology Journal, 4, 343–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei, T. , Ou, B. , Li, J. , Zhao, Y. , Guo, D. , Zhu, Y. et al (2013) Transcriptional profiling of rice early response to Magnaporthe oryzae identified OsWRKYs as important regulators in rice blast resistance. PLoS ONE, 8, e59720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao, S. , Ellwood, S. , Calis, O. , Patrick, E. , Li, T. , Coleman, M. et al (2001) Broad‐spectrum mildew resistance in Arabidopsis thaliana mediated by RPW8 . Science, 291, 118–120. [DOI] [PubMed] [Google Scholar]
- Xie, X. , He, Z. , Chen, N. , Tang, Z. , Wang, Q. and Cai, Y. (2019) The roles of environmental factors in regulation of oxidative stress in plant. Biomedical Research International, 2019, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao, H. , Wang, X. , Jia, Y. , Minkenberg, B. , Wheatley, M. , Fan, J. et al (2018) The rice blast resistance gene Ptr encodes an atypical protein and confers broad spectrum disease resistance. Nature Communications, 9, 2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
FIGURE S1 Confirmation of overexpression and knockout in Perox3 transgenic lines. (a) Determination of Perox3 RNA expression levels in the Perox3 overexpression plants. Leaf samples collected from Perox3 overexpression lines Perox3‐ox#1, Perox3‐ox#2, Perox3‐ox#3, and wild‐type TP309 at the seedling stage were subjected to RNA extraction followed by quantitative reverse transcription PCR (RT‐qPCR) analysis. RT‐qPCR results are normalized with the Ubq5 reference gene. Error bars represent SD from three replicates. Asterisks represent significant differences (*p < .01). (b) Confirmation of Perox3 knockout. The target site designed for knocking out the Perox3 gene by the CRISPR/Cas9 system. Verification of the knockout lines by PCR‐based sequencing. Two representative transgenic lines (abbreviated as Perox3‐KO#1 and Perox3‐KO#2) are generated from the TP309 genetic background
FIGURE S2 Cosegregation of enhanced susceptibility to Magnaporthe oryzae with the Perox3‐ox#1 transgene. (a) Photographs of representative leaves post punch inoculation. a1 to a10, and the TP309 control are shown. PCR‐based genotyping with a primer pair specific for the NptII gene was performed to determine whether the plants contained (+) or lacked (–) the Perox3‐ox construct. Blast isolate ZB15 was used for inoculations (conidial concentration of 5 × 105/ml). (b) Quantitation of lesion lengths from the segregants in (a)
TABLE S1 Primers used in this study
TABLE S2 The amplified part of each promoter of these genes used for one‐hybrid assay
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


